Poboljšanje stabilnosti probiotskog proizvoda sa Lactiplantibacillus plantarum 299v uvođenjem laminatnih („flow pack“) kesica Stručni rad
Glavni sadržaj članka
Apstrakt
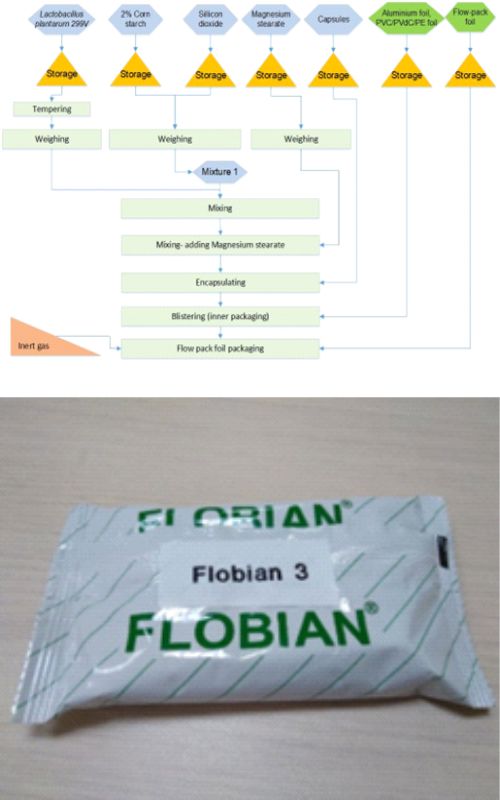
U svakodnevnoj upotrebi širom sveta sve su zastupljeniji probiotski proizvodi. Paraleno s tim, i u stručnoj javnosti raste interesovanje za istraživanja njihove proizvodnje i primene. Stabilnost probiotskog proizvoda se u farmaceutskoj proizvodnji obezbeđuje izborom probiotskog soja, formulacije i izborom pakovanja. Pakovanje je završna faza proizvodnje i presudni je faktor za stabilnost probiotika radi održanja vijabilnosti u toku roka upotrebe. U ovom radu opisan je izbor dodatnog pakovanja kapsuliranog probiotika Lactiplantibacillus plantarum 299v. Ispitivan je efekat dodatne zaštite blistera u laminatnim kesicama (engl. flow pack bag). Odabrani su blisteri napravljeni od PVC/PVdC/PE (polivinil hlorid/poliviniliden hlorid/polietilen) folije i aluminijumske folije, koji su zatim paraleno ispitani u poređenju sa blisterima pakovanim u dodatne kesice punjene inertnim gasom. Poređenjem vijabilnosti probiotskih ćelija laktobacila u uzorcima blistera u kesicama u odnosu na dva uzorka blistera bez tih kesica uočena je bolja zaštita probotskih ćelija od kiseonika, svetlosti i vlage u dodatnom pakovanju. Uvođenjem dodatne zaštite blistera u kesicama dobijena je za 11 % veća vijabilnost probiotika u poređenju sa uzorcima bez kesica posle 24 meseci što omogućava komformnije čuvanje proizvoda tokom upotrebe.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Aуторство-Nekomercijalno-Bez prerade 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Reference
WGO Review Team. World Gastroenterology Organisation Global Guidelines- Probiotics and prebiotics. World Gastroenterology Organisation; 2017 https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf
FAO (Food and Agriculture Organization of the United Nations), WHO (World Health Organization). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina; 2001. https://www.iqb.es/digestivo/pdfs/probioticos.pdf.
Salminen S, Ouwehand AC, Isolauri E. Clinical Applications of Probiotic Bacteria. Int Dairy J. 1998; 8(5-6): 563-572 https://doi.org/10.1016/S0958-6946(98)00077-6.
Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020; 70 (4): 2782-2858 https://doi.org/10.1099/ijsem.0.004107.
Korčok D, Tršić-Milanović N, Ivanović N, Đorđević B. Development of Probiotic Formulation for the Treatment of Iron Deficiency Anemia. Chem Pharm Bull. 2018; 66(4): 347–352 https://doi.org/10.1248/cpb.c17-00634.
Arvidsson Nordstrom E, Teixeira C, Montelius C, Jeppsson B, Larsson N. Lactiplantibacillus plantarum 299v (LP299V®): three decades of research. Benef Microbes. 2021; 12(5): 441-465 https://doi.org/10.3920/bm2020.0191.
Abdelazez A, Abdelmotaal H, Zong-Tao Z, Fang-Fang J, Sami R, Zhang L, Rahman Al Twaha A, Meng X. Potential benefits of Lactiplantibacillus plantarum as probiotic and its advantages in human health and industrial applications. Adv Environ Biol. 2018; 12(1): 16-27 http://dx.doi.org/10.22587/aeb.2018.12.1.4.
Axling U, Önning G, Martinsson Niskanen T, Larsson N, Hansson SR, Hulthén L. The effect of Lactiplantibacillus plantarum 299v together with low dose of iron on iron status in healthy pregnant women: A randomized clinical trial. Acta Obstet Gynecol Scand. 2021; 100(9): 1602–1610 https://doi.org/10.1111%2Faogs.14153.
Ipek G, Juneja V, Ahmedna M. Probiotics in food safety and human health. 1st ed., Boca Raton, FL, USA: Taylor and Francis Group; 2006 https://doi.org/10.1201/9781420027570.
Korčok D, Tršić- Milanović N, Ilić M, Mitić B, Đorđević B, Ivanović N. Improving the vability and stability of probiotic product with Saccharomyces boulardii DBVPG. Hem Ind. 2021; 75(1): 25-30 https://doi.org/10.2298/HEMIND201211008K.
Wang G, Chen Y, Xia Y, Song X, Ai L. Characteristics of Probiotic Preparations and Their Applications. Foods. 2022; 11: 2472 https://doi.org/10.3390/foods11162472.
Fenster K, Freeburg B, Holland C, Wong C, Rønhave Laursen R, Ouweghand AC. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms. 2019; 7(3): 83 https://doi.org/10.3390/microorganisms7030083.
Saarela M. Probiotic technology Maintaining viability and stability. Agro Food Industry Hi Tech. 2007; 18 (4): 19-21 https://www.researchgate.net/publication/289771526_Probiotic_technology_Maintaining_viability_and_stability.
Uddin M, Mamun A, Rashid M, Asaduzzaaman M. In-process and Finished Products Quality Control Tests for Pharmaceutical Capsules According to Pharmacopoeias. Int. J Pharm Res. 2015; 9(2): 1-9 https://doi.org/10.9734/bjpr%2F2016%2F22044.
Dao H, Lakhani P, Police A, Kallakunta V, Ajjarapu SS, Wu KW, Ponkshe P, Repka MA, Murthy SN. Microbial Stability of Pharmaceutical and Cosmetic Products. AAPS Pharm SciTech. 2018; 19(1): 60–78 https://doi.org/10.1208/s12249-017-0875-1.
Das S, Bhattacharjee D, Manna A, Basu S, Chowdhury S, Mukherjee S, Bhattacharyya BK. Effect of Different Excipients and packaging Materials on Commercial Preparation of Probiotic Formulation. Int J Pharm Sci Res. 2014; 5(5): 1830-1836 http://dx.doi.org/10.13040/IJPSR.0975-8232.5(5).1830-36.
Kolaček S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, Pot B, Shamir R, Szajewska H, Vandenplas Y, van Goudoever J, Weizman Z, and ESPGHAN Working Group for Probiotics and Prebiotics. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2017; 65(1): 117–124 https://doi.org/10.1097/mpg.0000000000001603.
De Man JC, Rogosa M, Elisabeth Sharpe M. A medium for the cultivation of lactobacilli. J Appl Microbiol. 1960; 23: 130-135 https://doi.org/10.1111/j.1365-2672.1960.tb00188.x.
Tranberg A, Klarin B, Johnson J, Pahlman LI. Efficacy of Lactiplantibacillus plantarum 299 and 299 v against nosocomial oropharyngeal pathogens in vitro and as an oral prophylactic treatment in a randomized, controlled clinical trial. Microbiologyopen. 2021; 10(1): 1151 https://doi.org/10.1002/mbo3.1151.
Sánchez Rodríguez M, Castán Urbano H, Muñoz de los Ríos D. Evaluation of Viability of Lactobacillus fermentum CECT 5716 in Gelatin and Gastroresistant Capsules. J Pharm Pharmacol. 2016; 4(8): 413-418 http://dx.doi.org/10.17265/2328-2150/2016.08.009.