Termodinamička svojstva dvokomponentnih smeša terpena i 1-propanola u intervalu temperature (288,15 – 323,15) K i na atmosferskom pritisku Original scientific paper
Glavni sadržaj članka
Apstrakt
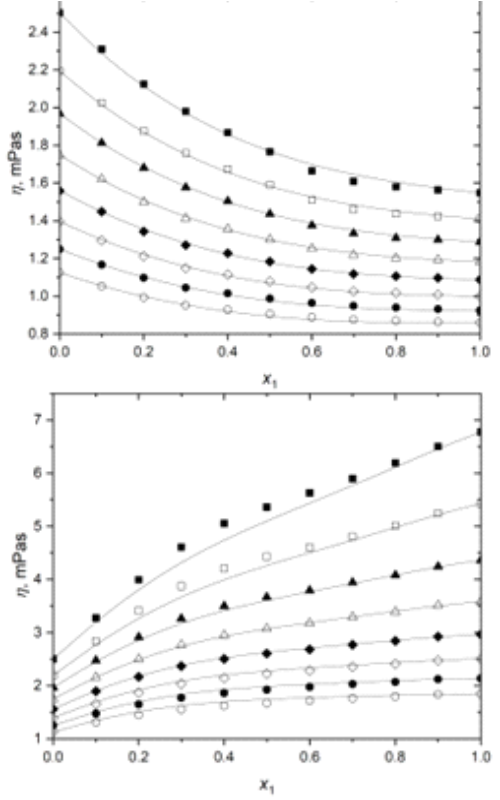
Najzastupljenija klasa hemijskih jedinjenja prisutna u esencijalnim uljima jesu terpeni. Smatraju se zelenim rastvaračima, a potiču iz prirodnih izvora poput biljaka, citrusnog voća, ali i lišća drveća ili šišarki. Pronalaze veliku komercijalnu upotrebu u granama prehrambene industrije, kao prirodne arome i dodaci hrani, pored čega su veoma zastupljeni u farmaceutskoj i kozmetičkoj industriji. U cilju proučavanja termodinamičkih svojstava smeša terpena (α-pinen, p-cimen i linalool) sa 1-propanolom, eksperimentalno su određene gustine i viskoznosti za navedene smeše. Eksperimentalna merenja rađena su u opsegu temperatura od 288,15 do 323,15 K na atmosferskom pritisku, za ceo opseg udela. Na osnovu eksperimentalno dobijenih rezultata za gustine i viskoznosti izračunate su vrednosti dopunske molarne zapremine, kao i vrednosti promene viskoznosti pri mešanju. Izmerene veličine (gustina i viskoznost) korelisane su korišćenjem Heric-Brewer-Jouyban-Acree modela, dok je za korelisanje dopunskih veličina (dopunska molarna zapremina i promena viskoznosti) korišćen Redlich-Kister polinom. Svi eksperimentalno dobijeni podaci i njihove izvedene veličine korišćene su za analizu neidealnog ponašanja odabranih smeša. Heric-Brewer-Jouyban-Acree model je uspešno korelisao eksperimentalne vrednosti za sva tri binarna sistema u celom temperaturnom opsegu i na atmosferskom pritisku, dok je Redlich-Kister uspešno korelisao izvedene veličine.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Aуторство-Nekomercijalno-Bez prerade 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministry of Scientific and Technological Development, Higher Education and Information Society,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-65/2024-03/200135
Reference
[1] Jiang Z, Kempinski C, Chappell J, Extraction and analysis of terpenes/terpenoids. Curr Protoc Plant Biol. 2006; 1(2): 345-358. https://doi.org/10.1002/cppb.20024
[2] Rodriguez-Garcia A, Hosseini S, Martinez-Chapa S, Cordell A, Multi-target activities of selected alkaloids and terpenoids. Mini-Rev Org Chem. 2017; 14(4): 272-279. 10.2174/1570193X14666170518151027
[3] Papada E, Gioxari A, Brieudes V, Amerikanou C, Halabalaki M, Skaltsounis L, Kaliora C. Bioavailability of terpenes and postprandial effect on human antioxidant potential. An open‐label study in healthy subjects. Mol Nut & Food Res. 2018; 62(3): 1700751. https://doi.org/10.1002/mnfr.201700751
[4] Allenspach M, Steuer C. α-Pinene: A never-ending story. Phytochem. 2021; 190: 112857. https://doi.org/10.1016/j.phytochem.2021.112857
[5] Jung K, Lee Y, Choi W, Jae J, Ha M, Suh J, Lee Y. Production of high-energy-density fuels by catalytic β-pinene dimerization: effects of the catalyst surface acidity and pore width on selective dimer production. Energy Conv and Manag. 2016; 116: 72-79. http://dx.doi.org/10.1016/j.enconman.2016.02.053
[6] Oswald P, Whitside R, Schäffer J, Köhler M. An experimental flow reactor study of the combustion kinetics of terpenoid jet fuel compounds: Farnesane, p-menthane and p-cymene. Fuel. 2017; 187: 43-50. http://dx.doi.org/10.1016/j.fuel.2016.09.035
[7] Balahbib A, El Omari N, Hachlafi E, Lakhdar F, El Menyiy N, Salhi N, Bouyahya A. Health beneficial and pharmacological properties of p-cymene. Food and Chem Tox. 2021; 153: 112259. https://doi.org/10.1016/j.fct.2021.112259
[8] Marchese A, Arciola R, Barbieri R, Silva S, Nabavi F, Tsetegho Sokeng J., Nabavi M. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials. 2017; 10(8): 947. https://doi.org/10.3390/ma10080947
[9] Cheng H, Lin Y, Yeh F, Cheng S, Chang T. Potential source of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaf: essential oil profile and enantiomeric purity. J Agric & Food Chem. 2012; 60(31): 7623-7628. https://doi.org/10.1021/jf302248w
[10] Hussain I, Anwar F, Sherazi H, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008; 108(3): 986-995. https://doi.org/10.1016/j.foodchem.2007.12.010
[11] Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food & Chem Toxic. 2008; 46(2): 446-475. https://doi.org/10.1016/j.fct.2007.09.106
[12] Meylemans A, Quintana L, Goldsmith R, Harvey G. Solvent‐free conversion of linalool to methylcyclopentadiene dimers: a route to renewable high‐density fuels. Chem Sus Chem. 2011; 4(4): 465-469. https://doi.org/10.1002/cssc.201100017
[13] Radovic I, Grozdanic N, Djordjevic B, Serbanovic S, Kijevcanin M. Prediction of excess molar volumes of binary mixtures by Prigogine-Flory-Patterson (PFP) and extended real association solution (ERAS) models, J Serb Chem Soc. 2017; 82: 1379-1390. http://dx.doi.org/10.2298/JSC170817103R
[14] Grozdanic N, Radovic I, Knezevic-Stevanovic A, Kijevcanin M. Volumetric properties of binary mixtures of tetrahydrofuran, dimethyl adipate, 1-butanol and 2-butanol from (288.15 to 323.15) K and modeling by Prigogine-Flory-Patterson (PFP) and Extended Real Association Solution (ERAS) models. J Mol Liq. 2021; 340: 117313. https://doi.org/10.1016/j.molliq.2021.117313
[15] Ilic-Pajic J, Radovic I, Grozdanic N, Stajic-Trosic J, Kijevcanin M. Volumetric and thermodynamic properties of binary mixtures of p-cymene with α-pinene, limonene and citral at atmospheric pressure and temperatures up to 323.15 K. Journal of Molecular Liquids. 2021; 344: 117486. https://doi.org/10.1016/j.molliq.2021.117486
[16] Ribeiro A, Bernardo-Gil G. Densities and refractive indices of components of pine resin. J Chem Eng Data. 1990; 35: 204-206. https://doi.org/10.1021/je00060a033
[17] Liao D-K, Meng X-L, Tong Z-F, Zheng D-X, Peng D-Y, Lu B. Excess Molar Enthalpies of p-cymene + α-Pinene + β-Pinene at (298.15, 308.15 and 318.15) K and at Atmospheric Pressure. J Chem Eng Data. 2007; 52: 808-811. http://dx.doi.org/10.1021/je060420p
[18] Clara R, Gomez Marigliano A, Solimo H. Density, Viscosity, and Refractive Index in the Range (283.15 to 353.15) K and Vapor Pressure of r-Pinene, d-Limonene, (()-Linalool, and Citral Over the Pressure Range 1.0 kPa Atmospheric Pressure. J Chem Eng Data. 2009; 54: 1087–1090. http://dx.doi.org/10.1021/je8007414
[19] Florido M, Andrade G, Capellini C, Carvalho H, Aracava K, Koshima C, Rodrigues C, Goncalves B. Viscosities and densities of systems involved in the deterpenation of essential oils by liquid-liquid extraction: New UNIFAC-VISCO parameters. J Chem Thermodyn. 2014; 72: 152-160. https://doi.org/10.1016/j.jct.2013.11.026
[20] Comelli F, Francesconi R, Castellari C. Densities, Viscosities, and Excess Molar Enthalpies of Binary Mixtures Containing Essential Oils at (298.15 and 313.15) K. The (S)-(−)-Limonene + Cineole, (S)-(−)-Limonene + Linalool, and Cineole + Linalool Systems. J Chem Eng Data. 2001; 46: 868–872. https://doi.org/10.1021/je010005r
[21] Comelli F, Ottani S, Francesconi R, Castellari C. Densities, Viscosities, and Refractive Indices of Binary Mixtures Containing n-Hexane + Components of Pine resins and Essential Oils at 298.15 K. J Chem Eng Data. 2002; 47: 93-97. https://doi.org/10.1021/je010216w
[22] Vercher E, Orchilles V, Miguel P, Martinez-Andreu A. Volumetric and Ultrasonic Studies of 1-Ethyl-3-methylimidazolium Trifluoromethanesulfonate Ionic Liquid with Methanol, Ethanol, 1-Propanol, and Water at Several Temperatures. J Chem Eng Data. 2007; 52: 1468-1482. https://doi.org/10.1021/je7001804
[23] Pal A, Kumar A. Viscosity of 1-Propanol + Ethylene Glycol Dimethyl, + Diethylene Glycol Dimethyl, + Triethylene Glycol Dimethyl, and + Tetraethylene Glycol Dimethyl Ethers at 288.15, 298.15 and 308.15 K. Ind J Chem A. 2003; 42: 2708-2716. https://nopr.niscpr.res.in/bitstream/123456789/20780/1/IJCA%2042A(11)%202708-2716.pdf
[24] Yang C, Lai H, Liu Z, Ma P. Density and Viscosity of Binary Mixtures of Diethyl Carbonate with Alcohols at (293.15 to 363.15) K and Predictive Results by UNIFAC-VISCO Group Contribution Method. J Chem Eng Data. 2006; 51: 1345-1351. http://dx.doi.org/10.1021/je0600808
[25] Rodriguez A, Canosa J, Dominguez A, Tojo J. Dynamic viscosities of diethyl carbonate with linear and secondary alcohols at several temperatures. J Chem Eng Data. 2004; 49: 157-162. http://dx.doi.org/10.1021/je0341413
[26] Saleh A, Habibullah M, Ahmed S, Uddin A, Uddin H, Khan M, Excess Molar Volumes and Viscosities of Some Alkanols with Cumene. Phys Chem Liq. 2006; 44: 31-43. http://dx.doi.org/10.1080/00319100500287853
[27] Jouyban A, Khoubnasabjafari M, Vaez-Gharamaleki Z, Fekari Z, Eugene Jr W. Calculation of the viscosity of binary liquids at various temperatures using Jouyban–Acree model. Chem and Pharm Bull. 2005; 53(5): 519-523. https://doi.org/10.1248/cpb.53.519
[28] Wan Normazlan D, Sairi A, Alias Y, Udaiyappan F, Jouyban A, Khoubnasabjafari M. Composition and temperature dependence of density, surface tension, and viscosity of EMIM DEP/MMIM DMP+ water+ 1-propanol/2-propanol ternary mixtures and their mathematical representation using the Jouyban–acree model. J Chem Eng Data. 2014; 59(8): 2337-2348. http://dx.doi.org/10.1021/je400576e
[29] Redlich O, Kister T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind & Eng Chem. 1948; 40(2): 345-348. https://doi.org/10.1021/ie50458a036