Fentonov tip oksidativne degradacije boje Orange G i binarnih smeša boja pomoću Oksona® aktiviranog katalizatorima na bazi aluminijum oksida dopiranih kobaltom Naučni rad
Glavni sadržaj članka
Apstrakt
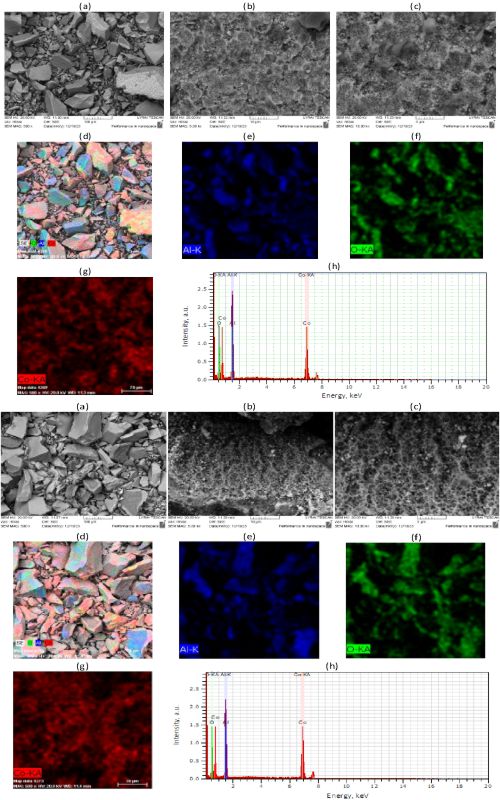
Dva kobaltom dopirana katalizatora na bazi aluminijum oksida, sa ra zličitim teksturalnim i strukturnim karakteristikama, dobijena su sol-gel postupkom sinteze, nakon koje su uzorci žareni na tempreturi od 1000 °C odnosno 1100 °C. Dobijeni materijali su ispitani kao katalizatori u procesu degradacije anjonske tekstilne boje Orange G (OG) uz korišćenje Oksona kao prekursora sulfatnih anjon radikala, koji su glavna oksidativna vrsta. Ispitan je uticaj temperature i početnog pH na efikasnost degradacije, i uočeno je da porast temperature povećava brzinu reakcije. Maksimalna efikasnost degradacijeje dobijena na 60 °C. Primenjeni su različiti kinetički modeli i pokazalo se da kinetički model pseudo-prvog reda najbolje opisuje kinetiku ispitanog procesa. Takođe je za oba katalizatora utvrđeno da je optimalna vrednost pH za ispitivanu reakciju u oblasti blizu neutralne. Koegzistirajući katjoni (Ca2+, Mg2+, K+ and Na+) i anjoni Clˉ and H2PO4ˉ su ubrzavali degradaciju OG, dok su je anjoni NO3ˉ, SO42ˉ i HCO3ˉ usporavali. Katalizatori su se pokazali efikasnii u degradaciji boja: metilensko plavo, osnovno plavo 41 i boje “Remazol brilliant blue”, kao i u degradaciji boja u binarnim smešama. Ipak, razlike u strukturnim i teksturalnim svojstvima su uticale na razlike u katalitičkoj efikasnosti ova dva katalizatora.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Aуторство-Nekomercijalno-Bez prerade 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200026
Reference
[1] Busca G. Structural, surface, and catalytic properties of aluminas. In: Jentoft F, ed. Advances in Catalysis, Norman, Oklahoma, USA, Elsevier Inc. 2014; 57: 2-412, ISSN 0360-0564; http://dx.doi.org/10.1016/B978-0-12-800127-1.00003-5
[2] Maldonado CS, De la Rosa JR, Lucio-Ortiz C, Hernández-Ramírez A, Castillón Barraza F, Valente J. Low concentration Fe-doped alumina catalysts using sol-gel and impregnation methods: The synthesis, characterization and catalytic performance during the combustion of trichloroethylene. Materials. 2014; 7: 2062-2086. http://dx.doi.org/10.3390/ma7032062
[3] Jiratova K, Beranek L, Properties of modified aluminas. Appl Catal. 1982; 2: 125-138. http://doi.org/10.1016/0166-9834(82)80196-6
[4] Matori KA, Wah LC, Hashim M, Ismail I, Mohd Zaid MH. Phase transformations of α-alumina made from waste aluminum via a precipitation technique., Int J Mol Sci. 2012; 13: 16812-16821 doi:10.3390/ijms131216812
[5] Gitzen WH. Alumina as a ceramic material; Wiley-American Ceramic Society, Columbus, OH, USA, 1970.
[6] Khattak AK, Afzal M, Saleem M, Yasmeen G, Ahmad R, Surface modifcation of alumina by metal doping, Colloid surf A 2000; 162: 99-106 https://doi.org/10.1016/S0927-7757(99)00218-6.
[7] Marinović S, Mudrinić T, Dojčinović B, Barudžija T, Banković P, Novaković T, Cobalt-doped alumina catalysts in catalytic oxidation of tartrazine induced by Oxone®. J Environ Chem Eng. 2021; 9: 106348 (8 pages). https://doi.org/10.1016/j.jece.2021.106348
[8] Jovanović A, Bugarčić M, Sokić M, Barudžija T, Pavićević V, Marinković A, Photodegradation of thiophanate-methyl under simulated sunlight by utilization of novel composite photocatalysts. Hem Ind. 2024. https://doi.org/10.2298/HEMIND230523004J
[9] Hu P, Long M, Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl Catal. 2016; 181: 103-117.http://dx.doi.org/10.1016/j.apcatb.2015.07.024
[10] Nfodzo P, Choi H, Triclosan decomposition by sulfate radicals: Effects of oxidant and metal doses. Chem Eng J. 2011; 174: 629-634.http://dx.doi.org/10.1016/j.cej.2011.09.076
[11] Zhou ZG, Du HM, Dai Z, Mu Y, Tong LL, Xing QJ, Liu SS, Ao Z, Zou JP, Degradation of organic pollutants by peroxymonosulfate activated by MnO2 with different crystalline structures: Catalytic performances and mechanisms. Chem Eng J. 2019; 374: 170-180. https://doi.org/10.1016/j.cej.2019.05.170
[12] Stevanović G, Jović-Jovičić N, Popović A, Dojčinović B, Milutinović-Nikolić A, Banković P, Ajduković M, Degradation of textile dyes by Oxone® activated by cobalt supported chitosan-derived carbon-smectite catalyst. Sci Sinter. 2024; https://doi.org/10.2298/SOS230427037S
[13] KulićMandić A, Bečelić Tomin M, PucarMilidrag G, Rašeta M, Kerkez Đ, Application of impregnated biocarbon produced from soybean hulls in dye decolorization. Hem Ind. 2021; 75: 307-320. https://doi.org/10.2298/HEMIND210427023K
[14] Ganesan S, Kokulnathan T, Sumathi S, Palaniappan A, Efficient photocatalytic degradation of textile dye pollutants using thermally exfoliated graphitic carbon nitride (TE-g-C3N4). Sci Rep. 2024; 14: 2284. https://doi.org/10.1038/s41598-024-52688-y
[15] Far HS, Hasanzadeh M, Najafi M, Rabbani M, Highly porous organoclay-supported bimetal-organic framework (CoNi-MOF/OC) composite with efficient and selective adsorption of organic dyes. Environ Sci Pollut Res. 2023; 30: 43714-43725
[16] https://doi.org/10.1007/s11356-023-25374-1
[17] Thao LT, Nguyen TV, Nguyen VQ, Phan NM, Ki Jae Kim, Huy NN, Dung NT, Orange G degradation by heterogeneous peroxymonosulfate activation based on magnetic MnFe2O4/α-MnO2 hybrid, J Environ Sci. 2023; 124: 379-396 https://doi.org/10.1016/j.jes.2021.10.008.
[18] Zhang J, Zhua MCL, Activation of peroxymonosulfate by iron-based catalysts for orange G degradation: role of hydroxylamine. RSC Adv. 2016; 6: 47562-47569 https://doi.org/10.1039/C6RA07231C
[19] Wu M, Wang Y, Lu B, Xiao B, Chen R, Liu H, Efficient activation of peroxymonosulfate and degradation of Orange G in iron phosphide prepared by pickling waste liquor. Chemosphere. 2021; 269: 129398.https://doi.org/10.1016/j.chemosphere.2020.129398
[20] Yu B, Li Z, Zhang S, Zero-valent copper-mediated peroxymonosulfate activation for efficient degradation of azo dye Orange G. Catalysts. 2022; 12: 700. https://doi.org/10.3390/catal12070700
[21] Madihi-Bidgoli S, Asghari F, Cheraghi S, Hamidinia H, Shagerdi E, AsadnezhadS, UV/periodate and UV/chlorine for dye degradation and real wastewater treatment: a comparative study, Water Pract Technol. 2023; 18: 2453-2468.https://doi.org/10.2166/wpt.2023.160
[22] Li C, Huang Y, Dong X, Sun Z, Duan X, Ren B, Zheng S, Dionysiou DD, Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine, ApplCatal B: Environ. 2019; 247: 10-23. https://doi.org/10.1016/j.apcatb.2019.01.079
[23] Zhou G, Xu Y, Zhang X, Sun Y, Wang C, Yu P, Efficient Activation of Peroxymonosulfate by Cobalt Supported Used Resin Based Carbon Ball Catalyst for the Degradation of Ibuprofen. Materials. 2022; 15:5003. https://doi.org/10.3390/ma15145003
[24] Li N, Wang Y, Cheng X, Dai H, Yan B, Chen G, Hou L, Wang S, Influences and mechanisms of phosphate ions onto persulfate activation and organic degradation in water treatment: A review, Water Res. 2022; 222: 118896. https://doi.org/10.1016/j.watres.2022.118896
[25] Sheng B, Huang Y, Wang Z, Yang F, AiL, Liu J,On peroxymonosulfate-based treatment of saline wastewater: When phosphate and chloride co-exist,RSC Adv.2018; 8: 13865. http://doi.org/10.1039/c8ra00600h
[26] Zhao X, Ana QD, Xiao ZY, Zhai SR, Shi Z, Seaweed-derived multifunctional nitrogen/cobalt-co doped carbonaceous beads for relatively high-efficient peroxymonosulfate activation for organic pollutants degradation, Chem Eng J. 2018; 353: 746-759. https://doi.org/10.1016/j.cej.2018.07.171
[27] Yuan R, Ramjaun SN, Wang Z, Liu J, Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds, J Hazard Mater. 2011; 196: 173-179. https://doi.org/10.1016/j.jhazmat.2011.09.007
[28] Xu A, Wei Y, Zou Q, Zhang W, Jin Y, Wang Z, Yang L, Li X, The effects of nonredox metal ions on the activation of peroxymonosulfate for organic pollutants degradation in aqueous solution with cobalt based catalysts: A new mechanism investigation, J Hazard Mater. 2020; 382: 121081. https://doi.org/10.1016/j.jhazmat.2019.121081
[29] Lončarević D, Dostanić J, Radonjić V, ŽivkovićLj, Jovanović D, Simultaneous photodegradation of two textile dyes usingTiO2 as a catalyst, React Kinet Mech Cat. 2016; 118: 153-164. http://doi.org/ 10.1007/s11144-016-0990-0
[30] Lin KYA, Lin TY, Degradation of Acid Azo Dyes Using Oxone Activated by Cobalt Titanate Perovskite, Water Air Soil Pollut. 2018; 229:10. https://doi.org/10.1007/s11270-017-3648-2
[31] Verma S, Rao BT, Singh R, Kaul R, Photocatalytic degradation kinetics of cationic and anionic dyes using Au-ZnO nanorods: Role of pH for selective and simultaneous degradation of binary dye mixtures, Ceram Int. 2021; 47: 34751-34764.https://doi.org/10.1016/j.ceramint.2021.09.014