Valorizacija kobalta iz istrošenih litijum-jonskih baterija luženjem u sistemima H2SO4-N2 i H2SO4-O2 i metodom elektrohemijskog taloženja Naučni rad
Glavni sadržaj članka
Apstrakt
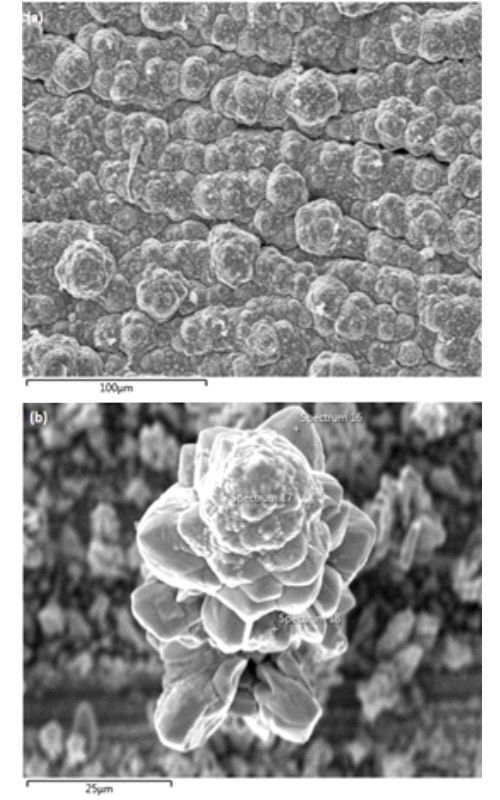
Tema rada je valorizacija kobalta iz katodnog materijala istrošenih litijum-jonskih baterija (LIBs) primenom metoda luženja i elektrohemijskog taloženja. Tokom eksperimenata luženja upoređivani su stepeni rastvaranja katodnog materijala u sistemima H2SO4-N2 i H2SO4-O2. Maksimalni stepeni ekstrakcije kobalta od 40 % u sistemu luženja H2SO4-N2 i 47 % u sistemu H2SO4-O2 postignuti su pod sledećim eksperimentalnim uslovima: koncentracija H2SO4 2 mol L-1, zapreminski protok azota/kiseonika 2 L min-1, koncentracija čvrste faze od 33 g L-1, i temperatura od 85 °C. Kinetika ekstrakcije kobalta iz katodnog materijala, u oba ispitivana sistema, bila je najpovoljnija u prvih 15 min, nakon čega je došlo do naglog smanjenja brzine reakcije. Kobalt je deponovan iz rastvora za luženje na bakarnu podlogu metodom galvanostatskog taloženja sa iskorišćenjem struje od 84 %. Potrošnja energije je bila 5.8 kWh kg-1 deponovanog kobalta. Metoda ciklične voltametrije (CV) korišćena je za određivanje potencijala taloženja kobalta, kao i sporednih reakcija koje se odvijaju u sistemu. Metodom skenirajuće elektronske mikroskopije sa energetsko disperzivnom spektrometrijom utvrđeno je da je tokom procesa elektrohemijskog taloženja kobalta došlo do aglomeracije čestica kobalta (u obliku karfiola) i da je kobalt deponovan u njegovom elementarnom stanju, što je potvrđeno rezultatima rendgenske difrakcione analize.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Aуторство-Nekomercijalno-Bez prerade 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/ 20013;451-03-47/2023-01/ 200052
Reference
Guo Y, Zhao YL, Lou X, Zhou T, Wang Z, Fang C, Guan J, Chen S, Xu X, Zhang RQ. Efficient degradation of industrial pollutants with sulfur (IV) mediated by LiCoO2 cathode powders of spent lithium ion batteries: A “treating waste with waste” strategy. J Hazard Mater. 2020; 399: 123090. https://doi.org/10.1016/j.jhazmat.2020.123090
Chen X, Ma H, Luo C, Zhou T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J Hazard Mater. 2017; 326: 77−86. https://doi.org/10.1016/j.jhazmat.2016.12.021
Wang MM, Zhang CC, Zhang FS. Recycling of spent lithium-ion battery with polyvinyl chloride by mechano chemical process. Waste Manage. 2017; 67: 232−239. https://doi.org/10.1016/j.wasman.2017.05.013
Meng Q, Zhang Y, Dong P, Liang F. A novel process for leaching of metals from LiNi1/3Co1/3Mn1/3O2 material of spent lithium ion batteries: Process optimization and kinetics aspects. J Ind Eng Chem. 2018; 61: 133−141. https://doi.org/10.1016/j.jiec.2017.12.010
Yang Y, Sun W, Bu Y, Zhang C, Song S, Hu Y. Recovering metal values from spent lithium ion battery via a combination of reduction thermal treatment and facile acid leaching. ACS Sustain Chem Eng. 2018; 6: 10445−10453. https://doi.org/10.1021/acssuschemeng.8b01805
Othman EA, Van der Ham AGJ, Miedema H, Kersten SRA. Recovery of metals from spent lithium-ion batteries using ionic liquid [P8888][Oleate]. Sep Purif Technol. 2020; 252: 117435. https://doi.org/10.1016/j.seppur.2020.117435
Prabaharan G, Barik SP, Kumar N, Kumar L. Electrochemical process for electrode material of spent lithium ion batteries. Waste Manage. 2017; 68: 527−533. https://doi.org/10.1016/j.wasman.2017.07.007
Patnaik P, Padhy SK, Tripathy BC, Bhattacharya IN, Paramguru RK. Electrodeposition of cobalt from aqueous sulfate solutions in the presence of tetra ethyl ammonium bromide. Trans Nonferrous Met Soc of China 2015; 25: 2047−2053. https://doi.org/10.1016/S1003-6326(15)63814-6
Garcia EM, Santos JS, Pereira EC, Freitas MBJG. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique. J Power Sources 2008; 185: 549−553. https://doi.org/10.1016/j.jpowsour.2008.07.011
Bhuiyan MS, Taylor BJ, Paranthaman M, Thompson JR, Sinclair JW. Microstructure and magnetic properties of electrodeposited cobalt films. J Mater Sci. 2008; 43: 1644−1649. https://doi.org/10.1007/s10853-007-2383-2
Rafsanjani-Abbasi A, Rahimi E, Shalchian H, Vahdati-Khaki J, Babakhani A, Hosseinpour S, Davoodi A. Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel. Materials 2018; 12: 90. https://doi.org/10.3390/ma12010090
Rigsby MA, Spurlin TA, Reid JD. The Multi-Functional Role of Boric Acid in Cobalt Electrodeposition and Superfill. J Electrochem Soc. 2020; 167: 112507. https://doi.org/10.1149/1945-7111/aba640
Santos JS, Trivinho-Strixino F, Pereira EC. Investigation of Co(OH)2 formation during cobalt electrodeposition using a chemometric procedure. Surf Coat Technol. 2010; 205: 2585−2589. https://doi.org/10.1016/j.surfcoat.2010.10.005
Zhou J, Wang SF, Song XS. Electrodeposition of cobalt in double-membrane three-compartment electrolytic reactor. Trans Nonferrous Met Soc of China 2016; 26: 1706−1713. https://doi.org/10.1016/S1003-6326(16)64279-6
Zech N, Landolt D. The influence of boric acid and sulfate ions on the hydrogen formation in Ni-Fe plating electrolytes. Electrochim Acta 2000; 45: 3461−3471. https://doi.org/10.1016/S0013-4686(00)00415-1
Ho HY, Chen WB, Fu TY, Chen SJ. On the Electrodepositing of Cobalt Nanoparticles on ITO in the Presence of Boric Acid. IEEE Trans Magn. 2014; 50: 2100304. https://doi.org/10.1109/TMAG.2013.2277758
Altimari P, Schiavi PG, Rubino A, Pagnanelli F. Electrodeposition of cobalt nanoparticles: An analysis of the mechanisms behind the deviation from three-dimensional diffusion-control. J Electroanal Chem. 2019; 851: 113413. https://doi.org/10.1016/j.jelechem.2019.113413
Medić D, Milić S, Alagić S, Đorđević I, Dimitrijević S. Classification of spent Li-ion batteries based on ICP-OES/X-ray characterization of the cathode materials. Hem Ind. 2020; 74: 221−230. https://doi.org/10.2298/HEMIND200114012M
Medić VD, Sokić MD, Nujkić MM, Đorđievski SS, Milić SM, Alagić ČS, Antonijević MA. Cobalt extraction from spent lithium-ion battery cathode material using a sulfuric acid solution containing SO2. J Mater Cycles Waste Manage. 2023; 25: 1008–1018. https://doi.org/10.1007/s10163-022-01580-w
Nayl AA, Elkhashab RA, Badawy SM, El-Khateeb MA. Acid leaching of mixed spent Li-ion batteries. Arab J Chem. 2017; 10: S3632−S3639. https://doi.org/10.1016/j.arabjc.2014.04.001
Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manage. 2013; 33: 1890−1897. https://doi.org/10.1016/j.wasman.2013.05.008
Jiang F, Chen Y, Ju S, Zhu Q, Zhang L, Peng J, Wang X, Miller JD. Ultrasound-assisted Leaching of Cobalt and Lithium from Spent Lithium-ion Batteries. Ultrason Sonochem. 2018; 48: 88−95. https://doi.org/10.1016/j.ultsonch.2018.05.019
Gao W, Zhang X, Zheng X, Lin X, Cao H, Zhang Y, Sun ZHI. Lithium Carbonate, Recovery from Cathode Scrap of Spent Lithium-ion Battery - a Closed-loop Process. Environ Sci Technol. 2017; 51: 1662−1669. https://doi.org/10.1021/acs.est.6b03320
Zhu SG, He WZ, Li GM, Zhou X, Zhang XJ, Huang JW. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans Nonferrous Met Soc of China 2012; 22: 2274−2281. https://doi.org/10.1016/S1003-6326(11)61460-X
Kaskiala T. Determination of oxygen solubility in aqueous sulfuric acid media. Miner Eng. 2002; 15: 853−857. https://doi.org/10.1016/S0892-6875(02)00089-4
Ndalamo J, Mulaba-Bafubiandi AF, Mamba BB. UV/visible spectroscopic analysis of CO3+ and CO2+ during the dissolution of cobalt from mixed Co-Cu oxidized ores. Int J Min Met and Mater. 2011; 18: 260−269. https://doi.org/10.1007/s12613-011-0432-y
Šupicová M, Rozik R, Trnková L, Oriňáková, Gálová M. Influence of boric acid on the electrochemical deposition of Ni. J Solid State Electrochem. 2006; 10: 61−68. https://doi.org/10.1007/s10008-005-0656-8
Metikoš-Huković M, Babić R. Passivation and corrosion behaviours of cobalt and cobalt–chromium–molybdenum alloy. Corros Sci. 2007; 49: 3570–3579. https://doi.org/10.1016/j.corsci.2007.03.023
Avramović Lj, Maksimović VM, Baščarević Z, Ignjatović N, Bugarin M, Marković R, Nikolić ND. Influence of the Shape of Copper Powder Particles on the Crystal Structure and Some Decisive Characteristics of the Metal Powders. Metals 2019; 9: 56. https://doi.org/10.3390/met9010056