Improving the stability of a probiotic product with Lactiplantibacillus plantarum 299v by introducing flow pack bags Technical paper
Main Article Content
Abstract
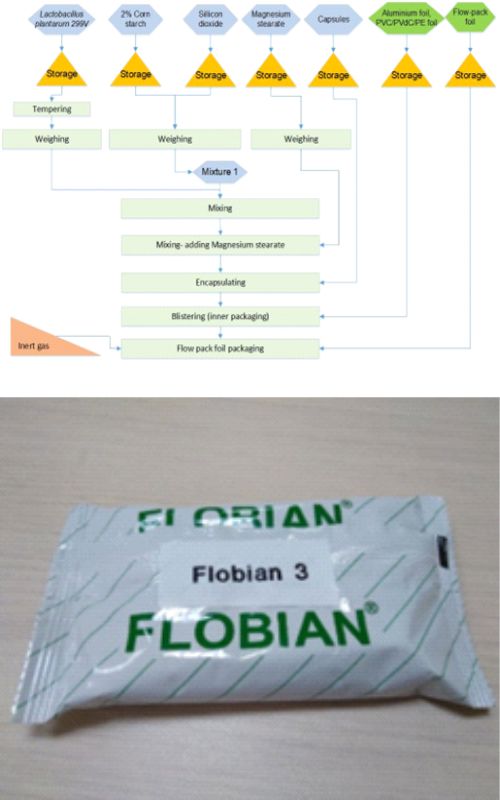
Probiotic products are becoming more common in everyday use around the world, while at the same time, the interest of scientists in researching probiotic production and use is increasing. Stability of a probiotic product in pharmaceutical production is affected by the choice of probiotic strain, formulation, and packaging. Packaging is the final stage of production and presents a crucial factor for the stability of probiotic products to maintain declared probiotic viability during the products' shelf life. The present research describes the influence of additional packaging material on the encapsulated probiotic product, which contains Lactiplantibacillus plantarum 299v. In specific, the effect of additional blister protection within flow pack bags was investigated. Blisters were made of a chloride/polyvinylidene chloride/polyethylene-triplex foil (PVC/PVdC/PE foil) and aluminum foil. Viability of probiotic lactobacilli cells protected in blisters only was compared to those packed in flow pack bags filled with nitrogen as an inert gas. Better protection of probiotic cells from oxygen, light, and moisture was determined in the capsules in the latter case. In specific, introduction of additional blister protection in flow pack bags resulted in ~11 % higher probiotic viability when compared to the other blister samples without such protection after 24 months, and therefore it enabled more efficient storage of the product during use.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
WGO Review Team. World Gastroenterology Organisation Global Guidelines- Probiotics and prebiotics. World Gastroenterology Organisation; 2017 https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf
FAO (Food and Agriculture Organization of the United Nations), WHO (World Health Organization). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina; 2001. https://www.iqb.es/digestivo/pdfs/probioticos.pdf.
Salminen S, Ouwehand AC, Isolauri E. Clinical Applications of Probiotic Bacteria. Int Dairy J. 1998; 8(5-6): 563-572 https://doi.org/10.1016/S0958-6946(98)00077-6.
Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020; 70 (4): 2782-2858 https://doi.org/10.1099/ijsem.0.004107.
Korčok D, Tršić-Milanović N, Ivanović N, Đorđević B. Development of Probiotic Formulation for the Treatment of Iron Deficiency Anemia. Chem Pharm Bull. 2018; 66(4): 347–352 https://doi.org/10.1248/cpb.c17-00634.
Arvidsson Nordstrom E, Teixeira C, Montelius C, Jeppsson B, Larsson N. Lactiplantibacillus plantarum 299v (LP299V®): three decades of research. Benef Microbes. 2021; 12(5): 441-465 https://doi.org/10.3920/bm2020.0191.
Abdelazez A, Abdelmotaal H, Zong-Tao Z, Fang-Fang J, Sami R, Zhang L, Rahman Al Twaha A, Meng X. Potential benefits of Lactiplantibacillus plantarum as probiotic and its advantages in human health and industrial applications. Adv Environ Biol. 2018; 12(1): 16-27 http://dx.doi.org/10.22587/aeb.2018.12.1.4.
Axling U, Önning G, Martinsson Niskanen T, Larsson N, Hansson SR, Hulthén L. The effect of Lactiplantibacillus plantarum 299v together with low dose of iron on iron status in healthy pregnant women: A randomized clinical trial. Acta Obstet Gynecol Scand. 2021; 100(9): 1602–1610 https://doi.org/10.1111%2Faogs.14153.
Ipek G, Juneja V, Ahmedna M. Probiotics in food safety and human health. 1st ed., Boca Raton, FL, USA: Taylor and Francis Group; 2006 https://doi.org/10.1201/9781420027570.
Korčok D, Tršić- Milanović N, Ilić M, Mitić B, Đorđević B, Ivanović N. Improving the vability and stability of probiotic product with Saccharomyces boulardii DBVPG. Hem Ind. 2021; 75(1): 25-30 https://doi.org/10.2298/HEMIND201211008K.
Wang G, Chen Y, Xia Y, Song X, Ai L. Characteristics of Probiotic Preparations and Their Applications. Foods. 2022; 11: 2472 https://doi.org/10.3390/foods11162472.
Fenster K, Freeburg B, Holland C, Wong C, Rønhave Laursen R, Ouweghand AC. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms. 2019; 7(3): 83 https://doi.org/10.3390/microorganisms7030083.
Saarela M. Probiotic technology Maintaining viability and stability. Agro Food Industry Hi Tech. 2007; 18 (4): 19-21 https://www.researchgate.net/publication/289771526_Probiotic_technology_Maintaining_viability_and_stability.
Uddin M, Mamun A, Rashid M, Asaduzzaaman M. In-process and Finished Products Quality Control Tests for Pharmaceutical Capsules According to Pharmacopoeias. Int. J Pharm Res. 2015; 9(2): 1-9 https://doi.org/10.9734/bjpr%2F2016%2F22044.
Dao H, Lakhani P, Police A, Kallakunta V, Ajjarapu SS, Wu KW, Ponkshe P, Repka MA, Murthy SN. Microbial Stability of Pharmaceutical and Cosmetic Products. AAPS Pharm SciTech. 2018; 19(1): 60–78 https://doi.org/10.1208/s12249-017-0875-1.
Das S, Bhattacharjee D, Manna A, Basu S, Chowdhury S, Mukherjee S, Bhattacharyya BK. Effect of Different Excipients and packaging Materials on Commercial Preparation of Probiotic Formulation. Int J Pharm Sci Res. 2014; 5(5): 1830-1836 http://dx.doi.org/10.13040/IJPSR.0975-8232.5(5).1830-36.
Kolaček S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, Pot B, Shamir R, Szajewska H, Vandenplas Y, van Goudoever J, Weizman Z, and ESPGHAN Working Group for Probiotics and Prebiotics. Commercial Probiotic Products: A Call for Improved Quality Control. A Position Paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2017; 65(1): 117–124 https://doi.org/10.1097/mpg.0000000000001603.
De Man JC, Rogosa M, Elisabeth Sharpe M. A medium for the cultivation of lactobacilli. J Appl Microbiol. 1960; 23: 130-135 https://doi.org/10.1111/j.1365-2672.1960.tb00188.x.
Tranberg A, Klarin B, Johnson J, Pahlman LI. Efficacy of Lactiplantibacillus plantarum 299 and 299 v against nosocomial oropharyngeal pathogens in vitro and as an oral prophylactic treatment in a randomized, controlled clinical trial. Microbiologyopen. 2021; 10(1): 1151 https://doi.org/10.1002/mbo3.1151.
Sánchez Rodríguez M, Castán Urbano H, Muñoz de los Ríos D. Evaluation of Viability of Lactobacillus fermentum CECT 5716 in Gelatin and Gastroresistant Capsules. J Pharm Pharmacol. 2016; 4(8): 413-418 http://dx.doi.org/10.17265/2328-2150/2016.08.009.