Desulphurisation of dibenzothiophene and 4,6 – dimethyl dibenzo¬thio-phene via enhanced hydrogenation reaction route using RePd–TiO2/SiO2 aerogel catalysts: Kinetic parameters estimation and modelling Original scientific paper
Main Article Content
Abstract
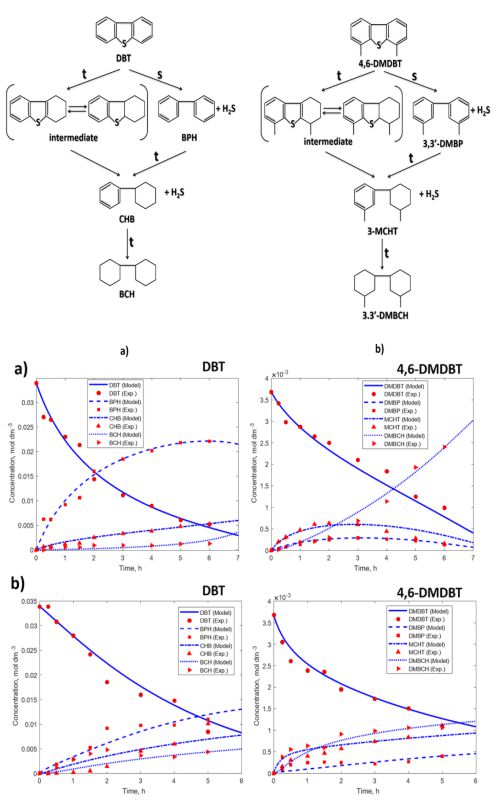
Re/Pd-TiO2/SiO2 aerogel catalysts were synthesized by using a sol-gel method and supercritical drying in excess solvent and investigated in the reaction of hydrodesulphurisation (HDS) of dibenzothiophene (DBT) and 4,6-dimethyl dibenzothiophene (4,6-DMDBT). Both Re/Pd catalysts, obtained with and without the use of mesitylene in the synthesis step, have shown increased conversions of up to 70 % in the desulphurization of 4,6-DMDBT, when compared to conventional Co/Mo hydroprocessing catalysts. This observation is of importance for conversion of highly refractory 4,6-DMDBT and hydroprocessing to produce ultra-low sulphur diesel fuels, ULSD. In order to quantify the extent of desulphurisation, which proceeds via a hydrogenation route, conversions of DBT and 4,6-DMDBT along with evolution of reaction products characteristic for the direct desulphurisation route and the hydrogenation route were monitored by using a gas chromatography–mass spectrometry (GC-MS) analytical technique. The reaction was performed at 630 K and 6 MPa in a batch catalytic reactor. The experimental results were used in the Hougen-Watson kinetic model describing DBT and 4,6-DMDBT desulphurisation on σ and τ active sites. Kinetic parameters of this complex catalytic kinetics were determined by using a Genetic Algorithm method and minimum deviation function. Values of calculated kinetic parameters and values of the ratio of 3-methylcyclohexyltoluene (MCHT and dimethyl biphenyl (DMBPH) expressed as the MCHT/(MCHT+DMBPH) ratio ranging between 0.66 and 0.94, have confirmed that the hydrogenation route is the dominant route for desulphurisation of 4,6-DMDBT.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200135
References
McGuiness L, Nunes MD, Kroes F, Chai I. Automated analysis of bauxite exploration drill hole samples by diffuse reflectance Fourier transform infrared (FTIR) spectroscopy. In: Proceedings of the 7th International Alumina Quality Workshop. Australia, 2005; 187–192.
Authier-Martin M, Forte G, Ostap S, See J. The mineralogy of bauxite for producing smelter-grade alumina. JOM 2001; 53(12) :36–40. https://doi.org/10.1007/s11837-001-0011-1
Nechitailov A P, Suss AG, Zhilina TI, Belanova E A. New method of analyzing bauxites to determine their main components and impurities. Metallurgist 2008; 52(11): 625–632. https://doi.org/10.1007/s11015-009-9104-9
Blagojevic D, Lazic D, Keselj D, Skundrić B, Dugic P, Ostojic G. Determining the content of silicon dioxide in bauxites using X-ray fluorescence spectrometry. Iran. J. Chem. Chem. Eng 2019; 38(4). https://doi.org/10.30492/IJCCE.2019.34231
Ostojic G, Lazic D, Zeljkovic S. Determination of the iron oxide content in bauxite: comparing ICP-OES with UV-VIS and volumetric analysis. Chem. Pap. 2020; 75(1): 389-396. https://doi.org/10.1007/s11696-020-01305-z
Idris N, Lahna K, Syamsuddin F, Ramli M. Study on Emission Spectral Lines of Iron, Fe in LaserInduced Breakdown Spectroscopy (LIBS) on Soil Samples. J. Phys. Conf. Ser 2017; 846(012020). https://doi.org/10.1088/1742-6596/846/1/012020
Carvalho AAC, Alves VC, Silvestre DM, Leme FO, Oliveira PV, Nomura CS. Comparison of FusedGlass Beads and Pressed Powder Pellets for the Quantitative Measurement of Al, Fe, Si and Ti in Bauxite by Laser Induced Breakdown Spectrometry (LIBS). Geostand. Geoanal. Res. 2017; 41(4): 585–592. https://doi.org/https://doi.org/10.1111/ggr.12173
Fahad M, Sajjad A, Shah KH, Shahzad A, Abrar M. Quantitative elemental analysis of high silica bauxite using calibration-free laser-induced breakdown spectroscopy. Appl. Opt. 2019; 58(27): 7588-7596. /https://doi.org/htps://doi.org/10.1364/AQ.58.007588
Upendra S, Mishra RS. Simultaneous multielemental analysis of alumina process samples using inductively coupled plasma spectrometry (ICP-AES). Anal. Chem.: Indian J. 2012; 11(1). https://www.tsijournals.com/articles/simultanious-multielemental-analysis-of-alumina-process-samples-using-inductively-coupled-plasma-spectrometry.pdf
Murray RW, Miller DJ, Krys KA. Analysis of major and trace elements in rocks, sediments, and interstitial waters by inductively coupled plasma-atomic emission spectrometry (ICP-AES). ODP Tech. Note, 2000; 29. https://doi.org/10.2973/ODP.TN.29.2000
SPECTRO - Smart Analyzer Vision Software, Online-Help vers. 5.01.09XX. Spectro Analytical Instruments GmbH. 2012
Giavarina D. Understanding Bland Altman analysis. Biochem. Medica 2015; 25(2), 141–151. https://doi.org/10.11613/BM.2015.015
Bilić-Zulle L. Comparison of methods: Passing and Bablok regression. Biochem. Medica 2011; 21 (1): 49–52. https://doi.org/10.11613/bm.2011.010
Medcalc manual, https://www.medcalc.org/manual/mountain-plot.php, Accessed May 23rd 2020
Linsinger T. Comparison of a measurement result with the certified value. Application Note 1, European Reference Materials. 2010; 1–2.
Certificate of Analysis, Standard Reference Material 697, bauxite Dominican, National Institute of Standards and Technology. 1991
Liberatore PA. Determination of Majors in Geological Samples by ICP-OES: Vol. ICP-AES Inst. 1993; 12 https://www.colby.edu/chemistry/CH332/laboratory/Geo-ICP-protocol2.pdf
Hauptkorn S, Pavel J, Seltner H. Determination of silicon in biological samples by ICP-OES after non-oxidative decomposition under alkaline conditions. Fresenius. J. Anal. Chem 2001; 370: 246–250. https://doi.org/10.1007/s002160100759
US. EPA Method 6010D (SW-846): Inductively coupled plasma-atomic emission spectrometry. Washington, DC, USA, 2014. https://www.epa.gov/esam/epa-method-6010d-sw-846-inductively-coupled-plasma-atomic-emission-spectrometry
Simundic AM. Statistical analysis in method comparison studies-Part one. 2016; www.acutecaretesting.org
AOAC (American Association Of Official Analytical Chemists), Appendix F : Guidelines for Standard Method Performance Requirements. 2016. https://www.aoac.org/resources/guidelines-for-standard-method-performance-requirements/
Taftazani A, Roto R, Ananda NR, Murniasih S. Comparison of NAA XRF and ICP-OES Methods on Analysis of Heavy Metals in Coals and Combustion Residues. Indones. J. Chem. 2017; 17(2): 228–237. https://doi.org/10.22146/ijc.17686
Rüdel H, Kӧsters J, Schӧrmann J. Determination of the Elemental Content of Environment Sample using ICP-OES, Guidelines for Chemical Analysis, Fraunhofer Institute for Molecular Biology and Applied Ecology, Schmallenberg, 2007. https://www.ime.fraunhofer.de/content/dam/ime/en/documents/AE/SOP_ICP-OES_en.pdf
Amorin A, Determination of major and minor elements in HF-digested soil samples using an Agilent 5110 ICP-OES, application note, Agilent Technologies.Inc. 2019. https://www.agilent.com/cs/library/applications/application_inert_sample_chamber_icp-oes_5110_5994-1213en_us_agilent.pdf
Krishna AK, Murthy NN, Govil PK. Multielement Analysis of Soils by Wavelength-Dispersive X-ray Fluorescence Spectrometry. At. Spectrosc 2007; 28(6): 202–214. https://www.researchgate.net/profile/Keshav-Krishna/publication/233786687_Multielement_Analysis_of_Soils_by_Wavelength-Dispersive_X-ray_Fluorescence_Spectrometry/links/57ecd91a08aebb1961ffc510/Multielement-Analysis-of-Soils-by-Wavelength-Dispersive-X-ray-Fluorescence-Spectrometry.pdf