Development and characterization of electrochemical sensors based on carbon modified with TiO2 nanoparticles Original scientific paper
Main Article Content
Abstract
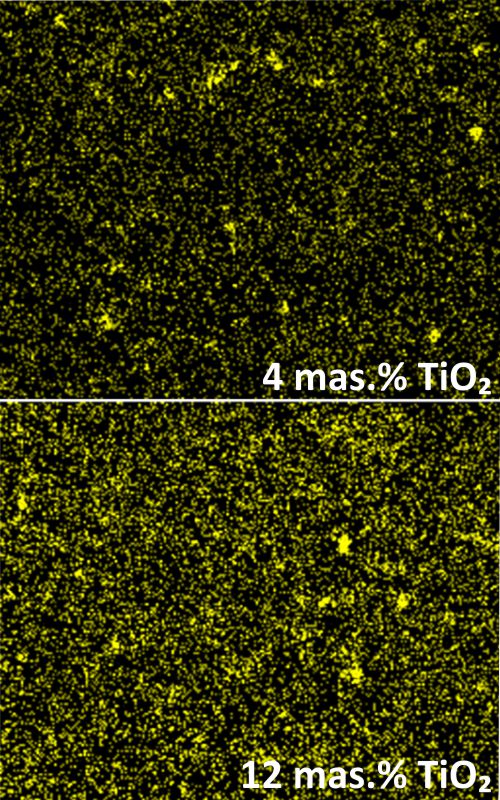
The aim of this study is the development and characterization of a carbon-based electrochemical sensor, modified with TiO2 nanoparticles for potential application in electroanalytical techniques. The influence of binder and modifier contents on morphological, physicochemical and electrochemical characteristics of the electrode material was investigated in order to determine the optimal ratio of the carbon material/binder/modifier. Carbon pastes were prepared from mixtures containing graphite powder, TiO2 nanoparticles and liquid hydrocarbons. Scanning electron microscopy showed that the electrode material becomes more compact with the addition and the increase in the binder material content, while increasing the proportion of TiO2 nanoparticles did not have any significant effect on the material morphology showing fairly homogeneous nanoparticle distribution in the graphite electrode material. The test results indicate that the modified carbon paste with 40 vol.% paraffin oil (PO) and 6-8 wt.% TiO2 nanoparticles is characterized by the lowest value of specific resistance. By applying cyclic voltammetry, the most pronounced degree of reversibility was obtained in relation to the standard reversible redox system ([Fe (CN)]-3/-4) for the electrode material with 30–40 vol.% PO and 8-10 wt.% TiO2 nanoparticles. Characterization of the electrode material based on carbon modified with TiO2 nanoparticles indicated that the optimal composition contains 40 vol.% PO and 6-8 wt.% TiO2 nanoparticles, which is important for application in electroanalytical techniques.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Švancara I, Walcarius A, Kalcher K, Vytřas K. Carbon paste electrodes in the new Millennium. Cent Eur J Chem. 2009; 7(4): 598-656 https://doi.org/10.2478/s11532-009-0097-9
Švancara I, Metelka R, Mikysek T, Vytřas K. 30 years with carbon paste electrodes at the University of Pardubice. SciPap. 2017; Series A 23: 5-50. https://hdl.handle.net/10195/75317
Guzsvány V, Papp Z, Švancara I, Vytras K. Insecticides - Advances in Integrated pest management. In: Insecticides – Advances in Integrated Pest Management. Rijeka, Hrvatska, 2012, pp. 541-578 https://doi.org/10.5772/2447
Švancara I, Kalcher K, Walcarius A, Vytřas K. Electroanalysis with Carbon Paste Electrodes. Boca Raton, CRC Press, Taylor&FrancisGroup; 2012 https://doi.org/10.1201/b11478
Luo X, Morrin A, Killard AJ, Smyth MR. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis. 2006; 18(4): 319–326 https://doi.org/10.1002/elan.200503415
Wongkaew N, Simsek M, Griesche Ch, Baeumner AJ. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem Rev. 2019; 119(1): 120–194 https://doi.org/10.1021/acs.chemrev.8b00172
Brainina Kh, Stozhko N, Bukharinova M, Vikulova E. Nanomaterials: Electrochemical Properties and Application in Sensor Phys Sci Rev. 2018; 3(9): 201880050 https://doi.org/10.1515/psr-2018-8050
Katz E, Willner I, Wang J. Electroanalytical and Bioelectroanalytical Systems Based on Metal and Semiconductor Nanoparticles. Electroanalysis. 2004; 16(1-2): 19-44 https://doi.org/10.1002/elan.200302930
Welch CM, Compton RG. Theuse of nanoparticles in electroanalysis: a review. Anal Bioanal Chem. 2006; 384(3): 601–619 https://doi.org/10.1007/s00216-005-0230-3
Zima J, Švancara I, Barek J, Vytras K. Recent advances in electroanalysis of organic and biological compounds at carbon paste electrodes. Crit Rev Anal Chem. 2009; 39: 204-227 https://doi.org/10.1080/10408340903011853
Kalcher K, Švancara I, Buzuk M, Vytras K, Walcarius A. Electrochemical sensors and biosensors based on heterogeneous carbon materials. MonatshChem. 2009; 140: 861-889 https://doi.org/10.1007/s00706-009-0131-9
Švancara I, Konvalina J, Schachl K, Kalcher K, Vytras K. Stripping voltammetric determination of iodide with synergistic accumulation at a carbon paste electrode. Electroanalysis. 1998; 10: 435-441 https://doi.org/10.1002/(SICI)1521-4109(199805)10:6%3C435::AID-ELAN435%3E3.0.CO;2-J
Bai J, Zhou B. Titanium Dioxide Nanomaterials for SensorApplications. Chem Rev. 2014; 114(19): 10131-10176 https://doi.org/10.1021/cr400625j
Xiaobo Chen, Samuel S Mao. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem Rev. 2007; 107: 2891-2959 https://doi.org/10.1021/cr0500535
Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, Glowacka-Sobotta A, Stanisz B, Goslinski T, Sobotta L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials. 2020; 10(2): 387 https://doi.org/10.3390/nano10020387
Mo SD, Ching WY. Electrical and optical properties of three phases of titanium dioxide: Rutile, anatase and brookite. Phys Rev B. 1995; 51(19): 13023-13032 https://doi.org/10.1103/PhysRevB.51.13023
Ansari SA, Khan MM, Ansari MO, Cho MH. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J Chem. 2016; 40(4): 3000-3009 https://doi.org/10.1039/C5NJ03478G
Babaei A, Moradi M , Sohrabi M, Feshki S, Marandi M. Fabrication of TiO2 Hollow Spheres and its Application in Modification of Carbon Paste Electrode For Simultaneous Determination of Dopamine and Piroxicam in the Presence of Ascorbic acid. J Nanostruct. 2018; 8(1):119-130. https://dx.doi.org/10.22052/JNS.2018.02.002
Ha TJ, Hong MH, Park CS, Park HH. Gas sensing properties of ordered mesoporous TiO2 film enhanced by thermal shock induced cracking. Sens Actuator B Chem. 2013; 181: 874-879 https://doi.org/10.1016/j.snb.2013.02.093
Fadillah G, Ariani F. A novel electrochemical synthesis of TiO2 nanoparticles and its application as bisphenol-B sensor. AIP Conference Proceedings 2021; 2370, 050001 https://doi.org/10.1063/5.0062211
Zarei E, Jamali MR, Bagheri J. Applicationof TiO2 Nanoparticles Modified Carbon Paste Electrode for the Determination of Vitamin B2. J Anal Chem. 2019; 74: 1213–1222 https://doi.org/10.1134/S1061934819120049
Narouei FH, Kirk KA, Andreescu S. Electrochemical Quantification of Lead Adsorption on TiO2 Nanoparticles. Electroanalysis. 2020; 33(1): 188-196 https://doi.org/10.1002/elan.202060152
Sarma M, Valle M. Improved Sensing of Capsaicin with TiO2 Nanoparticle Modified Epoxy Graphite Electrode. Electroanalysis. 2020; 32(2): 230-237 https://doi.org/10.1002/elan.201900400
OliveiraLuciana S, Alba Juan FG, SilvaValdinete L, RibeiroRogério T, FalcãoEduardo HL, Navarro M. The effect of surface functional groups on the performance of Graphite powders used as electrodes. J Electroanalytical Chem. 2018; 818: 106-113 https://doi.org/10.1016/j.jelechem.2018.04.022
Mikysek T, Stočes M, Švancara I, Ludvík J. Relation between the composition and properties of carbon nanotubes paste electrodes (CNTPEs). In: Vytřas K, Kalcher K, Švancara I. Sensing in Electroanalysis, Pardubice, Czech: University of Pardubice; 2010: 69-75 http://hdl.handle.net/10195/38243
Mikysek T, Stočes M, Švancara I, Ludvík J. The ohmic resistance effect for characterisation of carbon nanotube paste electrodes (CNTPEs). RSC Adv. 2012; 2: 3684-3690 https://doi.org/10.1039/C2RA20202F
Rabie Malha SI, Lahcen AA, Arduini F, Ourari A, Amine A. Electrochemical Characterization of Carbon Solid_like Paste Electrode Assembled Using Different Carbon Nanoparticles. Electroanalysis. 2015; 27: 1-9 https://doi.org/10.1002/elan.201500637
Ashrafi AM, Richtera L. Preparation and Characterization of Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes and Its Application in a Sensitive Assay of Antihyperlipidemic Simvastatin in Biological Samples. Molecules. 2019; 24: 2215 https://doi.org/10.3390/molecules24122215
Čović JS, Zarubica AR, Bojić AL, Troter TM, Ranđelović MS. Electrochemical study of novel composite electrodes based on glassy carbon bulk-modified with Pt and MoO2 nanoparticles supported onto multi-walled carbon nanotubes. J Serb Chem Soc. 2020; 85(9): 1185-1196 https://doi.org/10.2298/JSC200221043C
Andi Wang D, Chung DL. Dielectric and electrical conduction behavior of carbon paste electrochemical electrodes, with decoupling of carbon, electrolyte and interface contributions. Carbons. 2014; 72: 135-151 https://doi.org/10.1016/j.carbon.2014.01.066
Khodari M, Mersal GAM, Rabie EM, Assaf HF. Electrochemical Sensor based on Carbon Paste Electrode Modified by TiO2 nano-paricles for the Voltammetric Determination of Resorcinol. Int J Electrochem Sci. 2018; 13: 3460-3474 http://dx.doi.org/10.20964/2018.04.04
Lobón GS, Yepez A, Garcia LF, Morais RL, Vaz BG, Carvalho VV, Rodrigues de Oliveira GA, Luque R, Gil E. Efficient electrochemical remediation of microcystin-LR in tap water using designer TiO2@carbon electrodes. Sci Rep. 2017; 7: 41326 https://dx.doi.org/10.1038%2Fsrep41326
Khursheed A, Akbar M, Richa R, Shaikh MM. Construction of TiO2 nanosheets modified glassy carbon electrode (GCE/TiO2) for the detection of hydrazine. Mater Res Express. 2016; 3: 074005 http://dx.doi.org/10.1088/2053-1591/3/7/074005
Mashhadizadeh MH, Rasouli F. Design of a New Carbon Paste Electrode Modified with TiO2 Nanoparticles to Use in an Electrochemical Study of Codeine and Simultaneous Determination of Codeine and Acetaminophen in Human Plasma Serum Samples. Electroanalysis. 2014; 26: 2033–2042 https://doi.org/10.1002/elan.201400141
Mashhadizadeh MH, Afshar E. Electrochemical investigation of clozapine at TiO2 nanoparticles modified carbon paste electrode and simultaneous adsorptive voltammetric determination of two antipsychotic drugs. Electrochimica Acta. 2013; 87: 816-823 https://doi.org/10.1016/j.electacta.2012.09.004
Švancara I, Schachl. Testing of unmodified carbon paste electrodes. Chem Listy. 1999; 93: 490-499 http://www.chemicke-listy.cz/docs/full/1999_08_490-499.pdf
Mikysek T, Švancara I, Kalcher K, Bartoš M, Vytras K, Ludvík J. New approaches to the characterization of carbon paste electrodes using the ohmic resistance effect and qualitative carbon paste indexes. Anal Chem. 2009; 81(15): 6327-6333 http://dx.doi.org/10.1021/ac9004937
Jiang X, Manawan M, Feng T, Qian R, Zhao T, Zhou G, Kong F, Wang Q, Dai S, Pan JH. Anatase and rutil in evonik aeroxide P25: Heterojunctioned or individual nanoparticles. Catalysis Today. 2017; 300: 12-17 http://dx.doi.org/10.1016/j.cattod.2017.06.010
d Kiran Kumar RS, Mamatha GP, Muralidhara HB, Kumar KY, Prashanth MK, Electrochemical Studies of Dopamine Using Titanium Dioxide Nanoparticle Modified Carbon Paste Electrode, Anal Bioanal Electrochem. 2015; 7(2): 175-185 http://abechem.ir/No.%202-2015/2015,7(2)175-185.pdf
Piljac I. Senzori fizikalnih veličina i elektroanalitičke metode. Zagreb, Hrvatska: Mediaprint-Tiskara Hrastić d.o.o.; 2010 ISBN 978-953-95404-1-6
Hassaninejad-Darzi SK, Shajie F. A Sensitive Voltammetric Determination of Anti-Parkinson Drug Pramipexole Using Titanium Dioxide Nanoparticles Modified Carbon Paste Electrode. J Braz Chem Soc. 2016; 28(4): 529-539 http://dx.doi.org/10.5935/0103-5053.20160192
Tashkhourian J, Nami Ana SF, Hashemnia S, Hormozi-Nezhad MR. Construction of modified carbon paste electrode based on TiO2 nanoparticles for the determination of gallic acid. J Solid State Electrochem. 2013; 17: 157–165 https://doi.org/10.1007/s10008-012-1860-y
Garcia LF, Cunha CEPd, Moreno EKG, Thomaz DV, Sanz Lobón G, Luque R, Somerset V, De Souza Gil E. Nanostructured TiO2 Carbon Paste Based Sensor for Determination of Methyldopa. Pharmaceuticals. 2018; 11(4):99. http://dx.doi.org/10.3390/ph11040099
Radoman T, Džunuzović J, Jeremić K, Marinković A, Spasojević P, Popović I, Džunuzović E. Uticaj veličine nanočestica TIO2 i njihove površinske modifikacije na reološka svojstva alkidne smole. Hem Ind. 2013; 67(6): 923-932 https://doi.org/10.2298/HEMIND131106081R
Manjunatha KG, Kumara Swamy BE, Madhuchandra HD, Vishnumurthy KA. Synthesis, characterization and electrochemical studies of titanium oxide nanoparticle modified carbon paste electrode for the determination of paracetamol in presence of adrenaline. Chem Data Collec. 2021; 31: 100604 https://doi.org/10.1016/j.cdc.2020.100604
Tashkhourian J, Nami Ana SF, Hashemnia S, Hormozi-Nezhad MR. Construction of modified carbon paste electrode based on TiO2 nanoparticles for the determination of gallic acid. J Solid State Electrochem. 2013; 17: 157–165 https://doi.org/10.1007/s10008-012-1860-y
Akhond M, Absalan G, Tafakori A, Ershadifar H. Simultaneous Determination of Thiocyanateand Oxalate in Urine using a Carbon Ionic Liquid Electrode Modified with TiO2-Fe Nanoparticles. Anal Bioanal Chem. 2016; 3(1): 73-86 https://dx.doi.org/10.22036/abcr.2016.14554
Ardakani MM, Beitollahi H, Taleat Z, Niasari MS. Fabrication and characterization of molybdenum(VI) complex–TiO2 nanoparticles modified electrode for the electrocatalytic determination of L-cysteine. J Serb Chem Soc. 2011; 76(4): 575–589 http://dx.doi.org/10.2298/JSC100504042M
Merck KGaA, Darmstadt, Germany https://www.merckmillipore.com/INTL/en/product/Graphite,MDA_CHEM-104206 pristupljeno 11. 05. 2021.
Merck KGaA, Darmstadt, Germany https://www.merckmillipore.com/INTL/en/product/Paraffin,MDA_CHEM-107160 pristupljeno 11. 05. 2021.
Merck KGaA, Darmstadt, Germany https://www.merckmillipore.com/INTL/en/product/Tritolyl-phosphate,MDA_CHEM-814811 Pristupljeno 11. 05. 2021.
Xiongzhen Jiang, Maykel Manawan, Ting Feng, Ruifeng Qian, Ting Zhao, Guanda Zhou, Fantai Kong , Qing Wang, Songyuan Dai, Jia HongPan. Anatase and rutil in evonik aeroxide P25:Heterojunctioned or individual nanoparticles. Catalysis Today. 2017; 300: 12-17 http://dx.doi.org/10.1016/j.cattod.2017.06.010