The Effects of Poly(diallyldimethylammonium chloride) addition on the curing kinetics of urea-formaldehyde adhesives for particleboards Original scientific paper
Main Article Content
Abstract
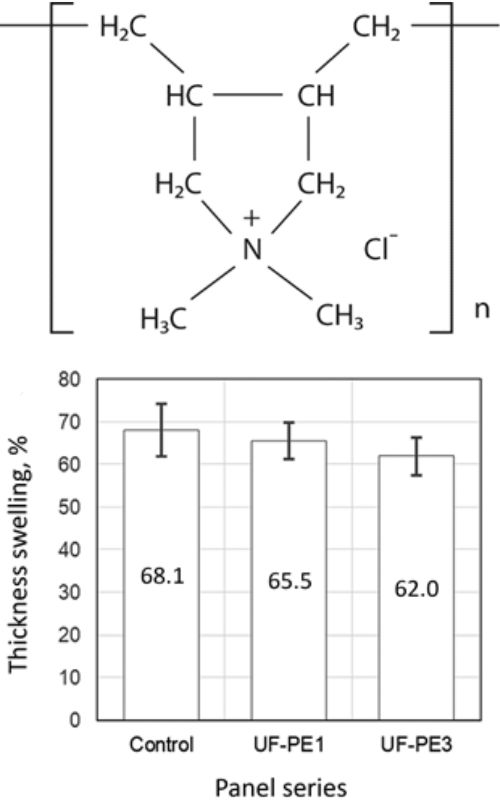
Addition of poly(diallyldimethylammonium chloride) (PDDA) on the performances of urea-formaldehyde (UF) adhesives was evaluated in this work. Three types of UF adhesives were prepared, one without PDDA addition, and two types with PDDA addition of 1 and 3 wt.% per dry UF adhesive mass. These UF adhesive systems were used for producing experimental particleboard panels. The addition of PDDA decreased the thickness swelling of the panel samples, while the internal bond of the particleboards increased significantly only at the highest PDDA content (3 wt.%). Differential scanning calorimetry (DSC) was applied to address the influence of PDDA on UF adhesive curing kinetics. DSC scans were performed in non-isothermal regimes using different heating rates (5, 10, and 20 °C∙min−1). The activation energy (Ea) of the curing reaction showed slightly lower values for the UF adhesive systems containing PDDA. However, the peak temperatures and enthalpy of reaction did not change significantly. The Kissinger-Akahira-Sunose and Friedman iso-conversional methods were applied to investigate the effects of PDDA addition on the UF adhesive curing process.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Li H, Wang L, Sheng K, Zou L, Ye B. Highly sensitive determination of esculetin on TiO2-NPs-coated poly(diallyl¬dimethyl¬amm-onium chloride)-functionalized graphene modified electrode. Talanta. 2016;161:838-846 https://doi.org/10.1016/j.talanta.2016.09.050
Wang B, Okoth OK, Yan K, Zhang J. A highly selective electrochemical sensor for 4-chlorophenol determination based on molecularly imprinted polymer and PDDA-functionalized graphene. Sensors Actuators, B Chem. 2016;236(236):294-303 https://doi.org/10.1016/j.snb.2016.06.017
Okoth OK, Yan K, Liu L, Zhang J. Simultaneous Electrochemical Determination of Paracetamol and Diclofenac Based on Poly(diallyldimethylammonium chloride) Functionalized Graphene. Electroanalysis. 2016;28(1):76-82 https://doi.org/10.1002/elan.201500360
Lu J, Liu Y, Liu X, Lu X, Liu X. Construction of a highly sensitive NADH sensing platform based on PDDA-rGO nanocomposite modified electrode. Ionics (Kiel). 2016;22(11):2225-2233 https://doi.org/10.1007/s11581-016-1753-7
Borowiec J, Yan K, Tin C-C, Zhang J. Synthesis of PDDA Functionalized Reduced Graphene Oxide Decorated with Gold Nanoparticles and Its Electrochemical Response toward Levofloxacin. J Electrochem Soc. 2015;162(3):H164-H169 https://doi.org/10.1149/2.0811503jes
Li F, Yang Q, Qiu F, Liu Y. Modification of superparamagnetic iron oxide nanoparticles with poly(diallyldimethylammonium chloride) at air atmosphere. Polym Adv Technol. 2016;27(11):1530-1534 https://doi.org/10.1002/pat.3834
Cho E, Won J. Novel composite membrane coated with a poly(diallyldimethylammonium chloride)/urushi semi-interpenetrating polymer network for non-aqueous redox flow battery application. J Power Sources. 2016;335:12-19 https://doi.org/10.1016/j.jpowsour.2016.10.020
Zhang J, Qiao J, Jiang G, Liu L, Liu Y. Cross-linked poly(vinyl alcohol)/poly (diallyldimethylammonium chloride) as anion-exchange membrane for fuel cell applications. J Power Sources. 2013;240:359-367 https://doi.org/10.1016/j.jpowsour.2013.03.162
Pandit S, Khilari S, Bera K, Pradhan D, Das D. Application of PVA-PDDA polymer electrolyte composite anion exchange membrane separator for improved bioelectricity production in a single chambered microbial fuel cell. Chem Eng J. 2014;257:138-147 https://doi.org/10.1016/j.cej.2014.06.077
Lin Z, Renneckar S, Hindman DP. Nanocomposite-based lignocellulosic fibers 1. Thermal stability of modified fibers with clay-polyelectrolyte multilayers. Cellulose. 2008;15(2):333-346 https://doi.org/10.1007/s10570-007-9188-y
Pillai K V., Renneckar S. Dynamic mechanical analysis of layer-by-layer cellulose nanocomposites. Ind Crops Prod. 2016;93:267-275 https://doi.org/10.1016/j.indcrop.2016.02.037
Zhang L, Chen H, Sun J, Shen J. Layer-by-layer deposition of poly(diallyldimethylammonium chloride) and sodium silicate multilayers on silica-sphere-coated substrate-facile method to prepare a superhydrophobic surface. Chem Mater. 2007;19(4):948-953 https://doi.org/10.1021/cm062535i
Sadeghi B, Pourahmad A. Synthesis of silver/poly (diallyldimethylammonium chloride) hybride nanocomposite. Adv Powder Technol. 2011;22(5):669-673 https://doi.org/10.1016/j.apt.2010.10.001
Huang J, Liu X, Thormann E. Surface Forces between Highly Charged Cationic Polyelectrolytes Adsorbed to Silica: How Control of pH and the Adsorbed Amount Determines the Net Surface Charge. Langmuir. 2018;34(25):7264-7271 https://doi.org/10.1021/acs.langmuir.8b00909
Zhang H, Zhao C, Li Z, Li J. The fiber charge measurement depending on the poly-DADMAC accessibility to cellulose fibers. Cellul 2015 231. 2015;23(1):163-173 https://doi.org/10.1007/s10570-015-0793-x
McLean D, Agarwal V, Stack K, Horne H, Richardson D. Synthesis of guar gum-graft-poly (acrylamide-co-diallyldimethylammonium chloride) and its application in the pulp and paper industry. BioResources. 2011;6(4):4168-4180 https://bioresources.cnr.ncsu.edu/resources/synthesis-of-guar-gum-graft-polyacrylamide-co-diallyldimethylammonium-chloride-and-its-application-in-the-pulp-and-paper-industry/
EN 827: Adhesives - Determination of conventional solids content and constant mass solids. 2005
EN 12092: Adhesives - Determination of viscosity. 2001
EN 1245: Adhesives - Determination of pH - Test method. 1998
EN 322: Wood-based panels - Determination of moisture content. 1993
EN 323: Wood-based panels - Determination of density. 1993
EN 317: Particleboards and fibreboards - Determination of swelling in thickness after. 1993
EN 319: Particleboards and fibreboards - Determination of tensile strength perpendicular to the plane of the board. 1993
Popović M, Popović J, Điporovic-Momčilović M, Vukić N, Budinski-Simendić J, Gavrilović-Grmuša I, Hamid F. The curing behavior of urea-formaldehyde adhesive in the presence of chemically treated narrow-leaved ash. Zast Mater. 2019;60(1):64-69 https://doi.org/10.5937/zasmat1901064P
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178-183 https://doi.org/10.1002/1096-987X(20010130)22:2<178::AID-JCC5>3.0.CO;2-%23
Kandelbauer A, Wuzella G, Mahendran A, Taudes I, Widsten P. Model-free kinetic analysis of melamine-formaldehyde resin cure. Chem Eng J. 2009;152(2-3):556-565 https://doi.org/10.1016/j.cej.2009.05.027
Sbirrazzuoli N, Vincent L, Mija A, Guigo N. Integral, differential and advanced isoconversional methods. Complex mechanisms and isothermal predicted conversion-time curves. Chemom Intell Lab Syst. 2009;96(2):219-226 https://doi.org/10.1016/j.chemolab.2009.02.002
Zhang C, Binienda WK, Zeng L, Ye X, Chen S. Kinetic study of the novolac resin curing process using model fitting and model-free methods. Thermochim Acta. 2011;523(1-2):63-69 https://doi.org/10.1016/j.tca.2011.04.033
Starink MJ. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404(1-2):163-176 https://doi.org/10.1016/S0040-6031(03)00144-8
EN 312: Particleboards - Specifications. 2010
Tischer S, Börnhorst M, Amsler J, Schoch G, Deutschmann O. Thermodynamics and reaction mechanism of urea decomposition. Phys Chem Chem Phys. 2019;21(30):16785-16797 https://doi.org/10.1039/C9CP01529A
Gao J, Zhao M, Qin J. Curing Kinetics of o-Cresol-formaldehyde Epoxy Resin/3-Methyl-tetrahydrophthalic Anhydride/Organic-Montmorillonite Nanocomposite by Isoconversional Methods. Iran Polym J. 2006;15(5):425-432 https://www.sid.ir/en/journal/ViewPaper.aspx?id=103253