Investigating possibilities for synthesis of novel sorbents and catalyst carriers based on ceramics with controlled open porosity Original scientific paper
Main Article Content
Abstract
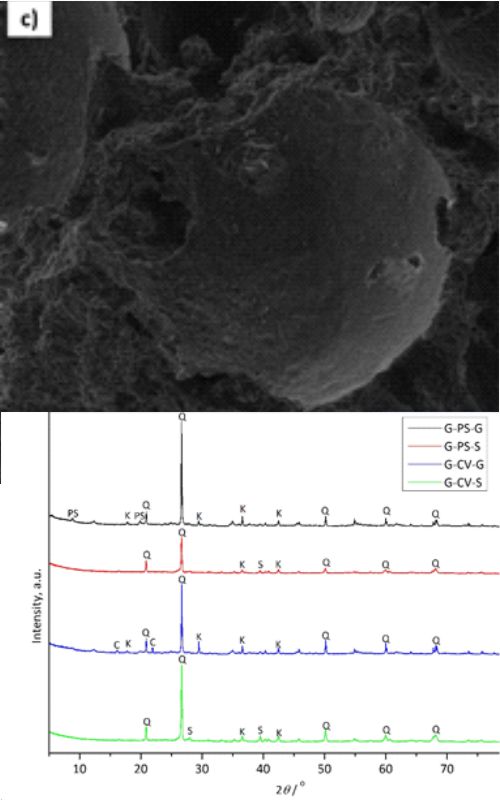
The aim of this study was to investigate a possibility of synthesis of porous ceramics with controlled open porosity, which could be used as sorbents and catalyst supports. Two organic additives were used to obtain open porosity: polystyrene beads and cellulose fibers, which are mixed with kaolin clay powder and the appropriate water content. Samples were sintered at 1050 oC for 1 h. Characterization of the obtained products included X-ray powder diffraction analysis (XRPD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA) and elemental CHNS analysis. In addition, porosity was examined by quantification of visual information. The specific surface areas were determined by the Brunauer–Emmett–Teller (BET) method. Also, density and compressive strength of the obtained samples were assessed. It was determined that by sintering, the organic component completely leaves the system. For samples prepared with polystyrene beads and with cellulose fibers, satisfactory mechanical properties were obtained: compressive strengths were 1.42 and 1.56 MPa, respectively. It was noted that significantly higher open porosity was obtained by using polystyrene beads as a sacrificial template (porosity of ~56 %) instead of cellulose fibers (porosity of ~6 %).
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200135; 451-03-9/2021-14/200288 and 451-03-68/2022-14/200288
References
World Health Organization (2011) Guidelines for Drinking-water Quality, 4th edn. World Health Organization, Geneva, http://www.who.int/guidelines/en/ Accessed June 15, 2021.
Ren Y, Yan N, Wen Q, Fan Z, Wei T, Zhang M, Ma J. Graphene/δ-MnO2 composite as adsorbent for the removal of nickel ions from wastewater. Chem Eng J. 2011; 175: 1–7. https://doi.org/10.1016/j.cej.2010.08.010
Hadi P, Barford J, McKay G. Synergistic effect in the simultaneous removal of binary cobalt-nickel heavy metals from effluents by a novel e-waste-derived material. Chem Eng J, 2013; 228: 140–146. https://doi.org/10.1016/j.cej.2013.04.086
Popović A, Rusmirović J, Radovanović Ž, Milošević M. Novel method of optimized synthesis of an efficient adsorbent based on vinyl modified lignin for cadmium (II) removal. In: Processing ’19, Society for Process Engineering within SMEITS. Belgrade, Serbia, 2019, pp. 195–201
Nikolić V, Kamberović Ž, Ranitović M, Gavrilovski M, Anđić Z. Synthesis of novel WO3/ZrSiO4 catalysts for dehalogenation of halogenated hydrocarbons. Metall Mater Eng. 2019; 25: 31-37. https://doi.org/10.30544/411
Lindon D. Montreal Protocol on Substances that Deplete the Ozone Layer, Volume 3 B, Report of the Task Force on Destruction Technologies. https://ozone.unep.org/treaties/montreal-protocol, Accessed June 15, 2021.
Pawnuk M, Grzelka A, Miller U, Sówka I. Prevention and Reduction of Odour Nuisance in Waste Management in the Context of the Current Legal and Technological Solutions. J Ecol Eng. 2020; 21: 34–41. https://doi.org/10.12911/22998993/125455
Cao J, Rambo CR, Sieber H. Preparation of Porous Al2O3-Ceramics by Biotemplating of Wood. J Porous Mater. 2004; 11: 163–172. https://doi.org/10.1023/B:JOPO.0000038012.58705.c9
Twigg MV, Richardson JT. Theory and applications of ceramic foam catalysts. Chem Eng Res Des. 2002; 80: 183–189. https://doi.org/10.1016/S0263-8762(02)72166-7
Damoah LNW, Zhang L. AlF3 reactive Al2O3 foam filter for the removal of dissolved impurities from molten aluminum: Preliminary results. Acta Mater. 2011; 59: 896–913. https://doi.org/10.1016/j.actamat.2010.09.064
Sokić M, Kamberović Ž, Nikolić V, Marković B, Korać M, Anđić Z, Gavrilovski M. Kinetics of NiO and NiCl2 hydrogen reduction as precursors and properties of produced Ni/Al2O3 and Ni-Pd/Al2O3 catalysts, Sci World J. 2015; 2015: 1-9. https://doi.org/10.1155/2015/601970
Drah A, Tomić NZ, Veličić Z, Marinković AD, Radovanović Ž, Veličković Z, Jančić-Heinemann R. Highly ordered macroporous γ-alumina prepared by a modified sol-gel method with a PMMA microsphere template for enhanced Pb2+, Ni2+ and Cd2+ removal. Ceram Int. 2017; 43: 13817–13827. https://doi.org/10.1016/j.ceramint.2017.07.102
Nikolić V, Kamberović Ž, Korać M, Anđić Z, Mihajlović A, Uljarević J. Nickel-based catalysts: Dependence of properties on nickel loading and modification with palladium. Hem Ind. 2016; 70: 137-142. https://doi.org/10.2298/HEMIND140928090N
Nor MAAM, Akil HM, Ahmad ZA. The effect of polymeric template density and solid loading on the properties of ceramic foam. Sci Sinter. 2009; 41: 319–327. https://doi.org/10.2298/SOS0903319N
Niesz K, Yang P, Somorjai GA. Sol-gel synthesis of ordered mesoporous alumina. Chem Commun. 2005; 1986–1987. https://doi.org/10.1039/b419249d
Deng ZY, Fukasawa T, Ando M, Zhang GJ, Ohji T. Microstructure and Mechanical Properties of Porous Alumina Ceramics Fabricated by the Decomposition of Aluminum Hydroxide. J Am Ceram Soc. 2001; 84: 2638–2644. https://doi.org/10.1111/j.1151-2916.2001.tb01065.x
de Faria CLL, de Oliveira TKR, dos Santos VL, Rosa CA, Ardisson JD, de Almeida Macêdo WA, Santos A. Usage of the sol-gel process on the fabrication of macroporous adsorbent activated-gamma alumina spheres, Microporous Mesoporous Mater. 2009; 120: 228–238. https://doi.org/10.1016/j.micromeso.2008.11.008
Nikolić V, Kamberović Ž, Anđić Z, Korać M, Sokić M. Synthesis of α-Al2O3 based foams with improved properties as catalyst carriers. Materiali in Tehnologije, 2014; 48: 45–50. http://mit.imt.si/izvodi/mit141/nikolic_v.pdf
Binner J, Ceramic foams, Cellular ceramics: Structure, Manufacturing, Properties and Applications. WILEY-VCH Verlag GmbH & Co. KGaA, Wienheim; 2005.
Bento AC, Kubaski ET, Sequinel T, Pianaro SA, Varela JA, Tebcherani SM. Glass foam of macroporosity using glass waste and sodium hydroxide as the foaming agent. Ceram Int. 2013; 39: 2423-2430. https://doi.org/10.1016/j.ceramint.2012.09.002
da Silva RC, Kubaski ET, Tenório-Neto ET, Lima-Tenório MK, Tebcherani SM. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda-lime glass matrix. Journal Non-Cryst. Solids, 2019; 511: 177-182. https://doi.org/10.1016/j.jnoncrysol.2019.02.003
da Silva RC, Kubaski ET, Tebcherani SM. Glass foams produced by glass waste, sodium hydroxide, and borax with several pore structures using factorial designs. Int J Appl Ceram Technol. 2019; 17: 75-83. https://doi.org/10.1111/ijac.13210
Yatsenko EA, Goltsman BM, Klimova LV, Yatsenko LA. Peculiarities of foam glass synthesis from natural silica-containing raw materials. J Therm Anal Calorim. 2020; 142: 119–127. https://doi.org/10.1007/s10973-020-10015-3
Wankasi D, Dikio ED. Comparative Study of Polystyrene and Polymethylmethacrylate Wastes as Adsorbents for Sorption of Pb2+ from Aqueous Solution. Asian J Chem. 2014; 24: 8295-8302. http://dx.doi.org/10.14233/ajchem.2014.16809
Ishak WHW, Ahmad I, Ramli S, Amin MCIM. Gamma Irradiation-Assisted Synthesis of Cellulose Nanocrystal-Reinforced Gelatin Hydrogels. Nanomaterials. 2018; 8: 749-762. http://dx.doi.org/10.3390/nano8100749
Milheiro FAC. Freire MN. Silva AGP. Holanda JNF. Densification behaviour of a red firing Brazilian kaolinitic clay. Ceram Int. 2005; 31: 757–763. http://dx.doi.org/10.1016/j.ceramint.2004.08.010
Castellano M, Turturro A, Riani P, Montanari T, Finocchio E, Ramis G, Busca G, 2010. Bulk and surface properties of commercial kaolins, Appl. Clay Science. 2010; 48: 446–454. http://dx.doi.org/10.1016/j.clay.2010.02.002
Hlavay J, Jonas K, Elek S, Inczedy J, Characterization of the particle size and the crystallinity of certain minerals by IR spectrophotometry and other instrumental methods-II. investigations on quartz and feldspar, Clays and Clay Minerals. 1978; 26: 139-143. https://doi.org/10.1346/CCMN.1978.0260209
Deju R, Mazilu C, Stanculescu I, Tuca C, Fourier transform infrared spectroscopic characterization of thermal treated kaolin, Rom Rep Phys. 2020; 72: 1-11. http://www.rrp.infim.ro/2020/AN72806.pdf
Poletto M, Ornaghi HL, Zattera AJ. Native Cellulose: Structure, Characterization and Thermal Properties. Mater. 2014; 7: 6105-6119. https://doi.org/10.3390/ma7096105
Hospodarova V, Singovszka E, Stevulova N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials, AJAC. 2019; 9: 303-310. http://dx.doi.org/10.4236/ajac.2018.96023
Akpinar S, Kusoglu IM, Ertugrul O, Onel K. Silicon carbide particle reinforced mullite composite foams, Ceram Int. 2012; 38: 6163-6169. https://doi.org/10.1016/j.ceramint.2012.04.067
Plesch G, Vargova M, Vogt UF, Gorbar M, Jesenak K. Zr doped anatase supported reticulated ceramic foams for photocatalytic water purification, Mat Res Bull. 2012; 47: 1680-1686. https://doi.org/10.1016/j.materresbull.2012.03.057