Removal of diesel pollution by biochar – support in water remediation Original scientific paper
Main Article Content
Abstract
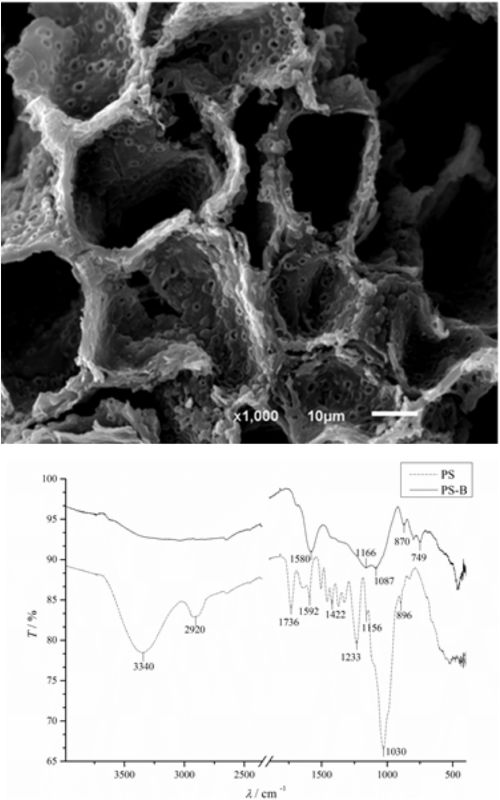
Water contaminated with diesel oil represents one of the greatest challenges in waste water management. Water soluble fraction (WSF) is of particular interest because of its toxicity to aquatic organisms and discharge regulations set by environmental authorities. Biochar sorbents have attracted great attention, due to their low cost origin and advantageous properties as well as high sorption capacities in sorption processes. In this study, we have reported the synthesis and characteristics of novel biochar sorbent made from waste lignocellulosic biomass (peach stones (PS)) and evaluated its possible application in removal of diesel WSF from synthetic water. Physiochemical characteristics of the biochar sample were analysed by scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET) method, and Fourier-transform infrared spectroscopy (FTIR), along with the elemental analysis. Characterisation of PS biochar (PS-B) indicated high multi porous surface area (159.1 m2 g-1) with the average pore diameter 2.7 nm. FTIR results indicated higher presence of aromatic compounds in PS-B as compared to PS. The sorption experiments performed in a batch system using PS-B resulted in more than 95 % removal of diesel WSF, reaching equilibrium after 5 h. Equilibrium data were well fitted by Freundlich isotherm, while the pseudo-second order equation fitted well the kinetic data, indicating chemisorption involving valency forces through the sharing/exchange of electrons between the sorbent and PS-B. Applications of ecotoxicology tests based on a microbial biosensor (Aliivibrio fischeri) have shown a significant toxicity reduction of water sample after the treatment with biochar.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Oil. https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/oil.html. Accessed August 10, 2021.
Pintor A, Vilar VJP, Botelho CMS, Boaventura RAR. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem Eng J. 2016; 297: 229-255 https://doi.org/10.1016/j.cej.2016.03.121.
Santos CA, Lenz D, Brandão GP, Chippari-Gomes AR, Gomes LC. Acute toxicity of the water-soluble fraction of diesel in Prochilodus vimboides Kner (Characiformes: Prochilodontidae). Neotropical Ichthyology. 2013; 11(1): 193-198 https://doi.org/10.1590/S1679-62252013000100022.
Srinivasan A, Viraraghavan T. Oil removal from water using biomaterials. Bioresour Technol. 2010; 101(17): 6594-6600 https://doi.org/10.1016/j.biortech.2010.03.079.
Khan E, Virojnagud W, Ratpukdi T. Use of biomass sorbents for oil removal from gas station runoff. Chemosphere. 2004; 57(7): 681–689 https://doi.org/10.1016/j.chemosphere.2004.06.028.
Huang Q, Song S, Chen Z, Hu B, Chen J, Wang X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar. 2019; 1: 45-73 https://doi.org/10.1007/s42773-019-00006-5.
Thompson KA, Shimabuku KK, Kearns JP, Knappe DRU, Summers RS, Cook SM. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ Sci Technol. 2016; 50(20): 11253-11262 https://doi.org/10.1021/acs.est.6b03239.
Lopičić Z, Stojanović M, Kaluđerović-Radoičić T, Milojković J, Petrović M, Mihajlović M, Kijevčanin M. Optimization of the process of Cu (II) sorption by mechanically treated Prunus persica L. - Contribution to sustainability in food processing industry. J Clean Prod. 2017; 156: 95-105 https://doi.org/10.1016/j.jclepro.2017.04.041.
Abdullah MA, Rahmah AU, Man Z. Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent. J Hazard Mater. 2010; 177(1-3): 683-691 https://doi.org/10.1016/j.jhazmat.2009.12.085.
Rouquerol F, Rouquerol J, Sing K. Adsorption by Powders and Porous Solids. 1975; London: Academic press.
EN ISO 9377: Water quality — Determination of hydrocarbon oil index — Part 2: Method using solvent extraction and gas chromatography. 2000
EN ISO 11348-3: Water quality-Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (luminescent bacteria test) - Part 3: Method using freeze-dried bacteria. 2007
Lopičić Z, Stojanović M, Marković S, Milojković J, Mihajlović, M, Kaluđerović-Radoičić T, Kijevčanin M. Effects of different mechanical treatments on structural changes of lignocellulosic waste biomass and subsequent Cu(II) removal kinetics. Arab J Chem. 2019; 12(8): 4091-4103 https://doi.org/10.1016/j.arabjc.2016.04.005.
Crombie K, Masek O, Sohi SP, Brownsort P, Cross A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy. 2012; 5(2): 122-131 https://doi.org/10.1111/gcbb.12030.
Zhang L, Ren Y, Xue Y, Cui Z, Wei Q, Han C, He J. Preparation of biochar by mango peel and its adsorption characteristics of Cd(II) in solution. RSC Adv. 2020; 10(59): 35878-35888 https://doi.org/10.1039/d0ra06586b.
Onorevoli B, Maciel G, Machado ME, Corbelini VA, Caramão EB, Jacques, R. Characterization of feedstock and biochar from energetic tobacco seed waste pyrolysis and potential application of biochar as an adsorbent. J Environ Chem Eng. 2018; 6(1): 1279-1287 https://doi.org/10.1016/j.jece.2018.01.039.
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph SD. Agronomic values of greenwaste biochar as a soil amendment. Aust J Soil Res. 2007; 45(8): 629-634 https://doi.org/10.1071/SR07109.
Joseph SD, Downie A, Munroe P, Crosky A, Lehmann J. Biochar for carbon sequestration, reduction of greenhouse gas emissions and enhancement of soil fertility: a review of the materials science. In: Proceedings of the Australian Combustion Symposium. Australia, 2007, pp 130–133.
Lopičić Z, Milojković J, Šoštarić T, Petrović M, Mihajlović M, Lačnjevac Č, Stojanović M. Influence of pH value on Cu (II) biosorption by lignocellulose peach shell waste material. Hem Ind. 2013; 67(6): 1007–1015 https://doi.org/10.2298/HEMIND121225018L.
Özçimen D, Ersoy-Meriçboyu A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew Energ. 2010; 35(6): 1319-1324 https://doi.org/10.1016/j.renene.2009.11.042.
Pehlivan E, Altun T, Cetin S, Iqbal BM. Lead sorption by waste biomass of hazelnut and almond shell. J Hazard Mater. 2009; 167(1-3): 1203–1208 https://doi.org/10.1016/j.jhazmat.2009.01.126.
Šoštarić T, Petrović M, Milojković J, Lačnjevac Č, Ćosović A, Stanojević M, Stojanović M. Application of apricot stone waste from fruit processing industry in environmental cleanup: copper biosorption study. Fruits. 2015; 70(5): 271-280 https://doi.org/10.1051/fruits/2015028.
Chonlong C, Mohini S, Wensheng Q. Lignin utilization: A review of lignin depolymerization from various aspects. Renew Sust Energ Rev. 2019; 107: 232-249 https://doi.org/10.1016/j.rser.2019.03.008.
Haiping Y, Rong Y, Hanping C, Dong HL, Chuguang Z. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007; 86(12-13): 1781-1788 https://doi.org/10.1016/j.fuel.2006.12.013.
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. Biochar effects on soil biota – A review. Soil Biol Biochem. 2011; 43(9): 1812-1836 https://doi.org/10.1016/j.soilbio.2011.04.022.
Obradović B. Guidelines for general adsorption kinetics modeling. Hem Ind. 2020; 74(1): 65-70 https://doi.org/10.2298/HEMIND200201006O.
Lagergren S, Svenska K. About the theory of so called adsorption of soluble substances. Veternskapsakad Handl. 1898; 24(4): 1-39.
Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999; 34: 451-465.
Low MJD. Kinetics of chemisorption of gases on solids. Chem Rev. 1960; 60: 267-312.
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM, Cheng M, Gong XM, Wan J, Luo H. Investigating the adsorption behaviour and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol. 2018; 261: 265–271 https://doi.org/10.1016/j.biortech.2018.04.032.
Cai L, Zhang Y, Zhou Y, Zhang X, Ji L, Song W, Zhang H, Liu J. Effective Adsorption of Diesel Oil by Crab-Shell-Derived Biochar Nanomaterials. Mater. 2019; 12(2): 236 https://doi.org/10.3390/ma12020236.
Barman SR, Das P, Mukhopadhayay A. Biochar from waste Sterculia foetida and its application as adsorbent for the treatment of PAH compounds: Batch and optimization. Fuel. 2021; 306: 121623 https://doi.org/10.1016/j.fuel.2021.121623.
Lowell S, Shields JE. Adsorption isotherms. In: Powder Surface Area and Porosity. Dordrecht, Germany, 1984, Springer.
Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906; 57: 385–470.
Sparks DL. Sorption Phenomena on Soils. In: Environmental Soil Chemistry, 2nd ed. Burlington: Academic Press; 2003: 133-186.
Foo KY, Hameed BH. Insights into the modelling of adsorption isotherm systems. Chem Eng J. 2010; 156(1): 2-10 https://doi.org/10.1016/j.cej.2009.09.013.
Antal MJ, Grønli M. The Art, Science, and Technology of Charcoal Production. Ind Eng Chem Res. 2003; 42(8): 1619−1640 https://doi.org/10.1021/ie0207919.
Muller JB, Melegari SP, Perreault F, Matias WG. Comparative assessment of acute and chronic ecotoxicity of water soluble fractions of diesel and biodiesel on Daphnia magna and Aliivibrio fischeri. Chemosphere. 2019; 221: 640-646 https://doi.org/10.1016/j.chemosphere.2019.01.069.
Hawrot-Paw M, Koniuszy A, Zając G, Szyszlak-Bargłowicz J. Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci Rep. 2020; 10: 16436 https://doi.org/10.1038/s41598-020-73469-3.
Abbas M, Adil M, Ehtisham-ul-Haque S, Munir B, Yameen M, Ghaffar A, Shar GA, Tahir MA, Iqbal M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci Total Environ. 2018; 626: 1295–1309 https://doi.org/10.1016/j.scitotenv.2018.01.066.