Investigation of the impact of mechanical activation on synthesis of the MgO-TiO2 system Technical paper
Main Article Content
Abstract
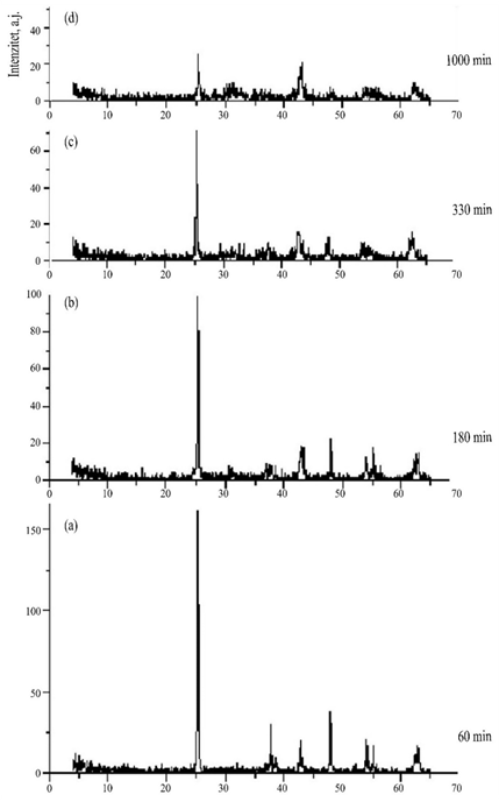
In this study, a mixture of magnesium oxide and titanium dioxide was mechanically activated in order to investigate the possibility of mechanochemical synthesis of magnesium titanate. Mechanical activation was performed for 1000 min in a high-energy vibro mill (type MH954/3, KHD Humboldt Wedag AG, Germany). The mill is equipped with housing having a horizontally placed shutter. The cylindrical stainless steel working vessel, with inner dimensions of 40 mm in height and 170 mm in diameter, has working elements consisting of two free concentric stainless steel rings with a total weight of 3 kg. The engine power is 0.8 kW. Respecting the optimal amount of powder to be activated of 50-150 g and the stoichiometric ratio of the reactants in the equation presenting the chemical reaction of magnesium titanate synthesis, the starting amounts were 20.2 g (0.5 mol) of MgO and 39.9 g (0.5 mol) TiO2. During the experiments, X-ray diffraction analysis of the samples taken from the reaction system after 60, 180, 330, and 1000 min of mechanical activation was performed. Atomic absorption spectrophotometry was used for chemical composition analysis of samples taken at different activation times. Based on the X-ray diffraction analysis results, it can be concluded that the greatest changes in the system took place at the very beginning of the mechanical activation due to the disturbance of the crystal structure of the initial components. X-ray diffraction analysis of the sample after 1000 min of activation showed complete amorphization of the mixture, but diffraction maxima characteristic for magnesium titanate were not identified. Therefore, the mechanical activation experiments were stopped. Evidently, the energy input was not sufficient to overcome the energy barrier to form a new chemical compound - magnesium titanate. The failure to synthesize magnesium titanate is explained by the low negative Gibbs energy value of
-25.8 kJ/mol (despite the theoretical possibility that the reaction will happen), as well as by the amount of mechanical energy entered into the system during activation which was insufficient to obtain the reaction product. Although the synthesis of MgTiO3 was not achieved, significant results were obtained which identify models for further investigations of the possibility of mechanochemical reactions of alkaline earth metals and titanium dioxide.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Tyliszczak B, Gaca K, Sobczak-Kupiec A, Dulian P. Mechanochemical synthesis and investigations of calcium titanate powders and their acrylic dispersions. J Eur Ceram Soc. 2014; 34(10): 2259-2264.
Cherdchom S, Rattanaphan T, Chanadee T. Calcium Titanate from food waste: Combustion Synthesis, Sintering, Characterization, and Properties. Adv Mater Sci Eng. 2019; 9639016.
Fauzi F, Habieb AM, Noviyanto A, Kusumaningrum R, Sukmarani G, Muhammad E, Widodo V, Amalia D, Aryanto D, Rochman N. The Effect of Mechanochemical on The Formation of Calcium Titanate (CaTiO3) Prepared by High Energy Milling. In: Proceedings of IOP Conference Series: Materials Science and Engineering, International Conference on Advanced Materials and Technology. Indonesia, Bogor, 2019, 924, pp. 8-9.
Manafi S, Jafarian M. Synthesis of perovskite CaTiO3 nanopowders with different morphologies by mechanical alloying without heat treatment. Int J Phys Sci. 2013; 8(23): 1277-1283.
Guomin M, Murakami Y, Shindo D, Saito F. Mechanochemical synthesis of CaTiO3 from a CaO–TiO2 mixture and its HR-TEM observation. Powder Technol. 1999; 105(1–3): 162-166.
Branković G, Vukotić V, Branković Z, Varela J. Investigation on possibility of mechanochemical synthesis of CaTiO3 from different precursors. J Eur Ceram Soc. 2007; 27(2–3): 729-732.
Palaniandy S, Jamil N. Influence of milling conditions on the mechanochemical synthesis of CaTiO3 nanoparticles. J Alloy Compd. 2009; 476(1–2): 894-902.
Tyliszczak B, Gaca K, Sobczak-Kupiec A, Dulian P. Mechanochemical synthesis and investigations of calcium titanate powders and their acrylic dispersions. J Eur Ceram Soc. 2014; 34(10): 2259-2264.
Wieczorek-Ciurowa K, Dulian P, Nosal A, Domagała J. Effects of reagents nature on mechanochemical synthesis of calcium titanate. J Therm Anal Calorim. 2010; 101(2): 471–477.
Ralphs K, Hardacreand C, James S. Application of heterogeneous catalysts prepared by mechanochemical synthesis. Chem Soc Rev. 2013; 42: 7701-7718.
Lazarević Z, Bobić J, Romčević N, Paunović N, Stojanović B. Study of Barium Bismuth Titanate Prepared by Mechanochemical Synthesis. Sci Sinter. 2009; 41: 329-335.
Đorđević N, Obradović N, Filipović S. Kinetika mehanohemijske sinteze barijum-titanata, Tehnika. 2011; 66(3): 367-371.
Li X, Shih W. Size Effects in Barium Titanate Particles and Clusters. J Am Ceram Soc. 1997; 80(11): 2844-2852.
Obradović N, Filipović S, Pavlović V, Mitrić M, Marković S, Mitić V, Đorđević N, Ristić M. Isothermal sintering of barium-zinc-titanate ceramics. Ceram Int. 2011; 37(1): 21-27.
Pavlović V, Nikolić M, Nikolić Z, Branković G, Živković Lj, Pavlović V, Ristić M. Microstructural evolution and electric properties of mechanically activated BaTiO3 ceramics. J Eur Ceram Soc. 2007; 27(2-3): 575-579.
Stojanović B, Pavlović V, Pavlović VP, Đurić S, Marinković B, Ristić M. Dielectric properties of barium-titanate sintered from tribophysically activated powders. J Eur Ceram Soc. 1999; 19 (6-7): 1081-1083.
Tkacova K. Mechanical activation of minerals. Amsterdam, New York, Elsevier; 1989.
Yangyun S, Brook RJ. Mechanism of reactive sintering of aluminium nitride. Sci Sinter. 1985; 17: 35-47.
Zdujic M, Poleti D, Jovalekic C, Karanovic L. The evolution of structure induced by intensive milling in the system 2Bi(2)O(3) center dot 3TiO(2). J Non-Cryst Solids. 2006; 352(28-29): 3058-3068.
Bobić J, Vijatović M, Rojac T, Stojanović B. Characterization and properties of barium bismuth titanate. Process Appl Ceram. 2009; 3(1-2): 9–12.
Stojanovic B, Paiva-Santos C, Cilense M, C. Valekic J, Lazarevic Z. Structure study of Bi4Ti3O12 produced via mechanochemically assisted synthesis. Mater Res Bull. 2008; 43: 1743–1753.
Kudłacik-Kramarczyk S, Drabczyk A, Głąb M, Dulian P, Bogucki R, Miernik K, Sobczak-Kupiec A, Tyliszczak B. Mechanochemical Synthesis of BaTiO3 Powders and Evaluation of Their Acrylic Dispersions. Materials. 2020; 13(15): 3275.
Buscaglia V, Randall C. Size and scaling effects in barium titanate. J Eur Ceram Soc. 2020; 40: 3744–3758.
Barber P, Balasubramanian S, Anguchamy Y, Gong S, Wibowo A, Gao H, Ploehn H, Loye H. Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage. Materials. 2009; 2:1697–1733.
Wei X, Liu Y, Zhao D, Ge S. 3D printing of piezoelectric barium tinatate with high density from milled powders. J Eur Ceram Soc. 2020; 40(15): 5423-5430
Ziegmann A, Schubert D. Influence of the particle size and the filing degree of barium titanate filled silicone elastomers used as potential dielectric elastomers on the mechanical properties and the crosslinking density. Mater Today Commun. 2018; 14: 90–98.
Berbenni V, Mariniv A, Bruni G. Effect of Mechanical Activation on the Preparation of SrTiO3 and Sr2TiO4 Ceramics from the Solid State Systems SrCO3–TiO2. J Alloy Compd. 2001; 329(1–2): 230–238.
Wang J, Yin S, Zhang Q, Saito F, Sato T. Mechanochemical synthesis of SrTiO3−xFx with high visible light photocatalytic activities for nitrogen monoxide destruction. J Mater Chem. 2003; 13: 2348-2352.
Wang J, Yin S, Zhang Q, Saito F, Sato T. Mechanochemical Synthesis and Photocatalytic Activity of Nitrogen Doped SrTiO3. J Ceram Soc Jpn., 2004; 112(5): 1408-1410.
Živojinović J, Pavlović V, Kosanović D, Marković S, Krstić J, Blagojević V, Pavlović V. The influence of mechanical activation on structural evolution of nanocrystalline SrTiO3 powders. J Alloy Compd. 2017; 695: 863-870.
Wang TX, Liu SZ, Chen J. Molten salt synthesis of SrTiO3 nanocrystals using nanocrystalline TiO2 as a precursor. Powder Technol. 2011; 205: 289–291.
Avvakumov EG. Mekhanicheskie metody aktivacii khimicheskih procesov, Moskva, SSSR, Akademii Nauk; 1986.
Boldyrev VV. Mechanochemistry and mechanical activation of solids. Solid State Ionics, 1993; 63-65: 537-543.
Glasstone S. Textbook of Physical Chemistry. Lancaster, PA Lancaster Press; 1967.
Filipovic S, Obradovic N, Pavlović V, Markovic S, Mitrić M, Ristic M. Influence of Mechanical Activation on Microstructure and Crystal Structure of Sintered MgO-TiO2 System. Sci Sinter. 2010; 42(2): 143-151.
Filipovic S, Obradovic N, Kosanovic D, Pavlovica V, Djordjevic A. Sintering of the mechanically activated MgO-TiO2 system. J Ceram Process Res. 2013; 14(1): 31-34.
Nikzad L, Ghofrani S, Majidian H, Ebadzadeh T. Effect of Ball Milling on Reactive Microwave Sintering of MgO-TiO2 System. ACERP. 2016; 2(3): 25-28.
Khalajabadi S, Rafiq M, Kadir A, Izman S, Bakhsheshi-Rad H, Farahany S. Effect of mechanical alloying on the phase evolution, microstructure and bio-corrosion properties of a Mg/HA/TiO2/MgO nanocomposite. Ceram Int. 2014; 40(10): 16743-16759.
Bhuyan R, Sahoo P, Basanta K, Sarangi A. Structural and Thermal Study of Mg2TiO4 Nanoparticles Synthesized by Mechanical Alloying Method. Micro Nanosyst. 2020; 12(2): 87-91.
Yang H, Zhihong L, Yumei Z. Effect of MgO–TiO2–SiO2 additions on in-situ anisotropic grains growth and mechanical properties of corundum abrasive using pseudo-boehmite as raw material. Ceram Int. 2020; 46 (2): 1934-1939.
Khalajabadi S, Kadir M, Izman S, Yusop M. Facile fabrication of hydrophobic surfaces on mechanically alloyed-Mg/HA/TiO2/MgO bionanocomposites. Appl. Surf. Sci. 2015; 324: 380-392.
Vidojković V. Proučavanje mehanizma i kinetike mehanohemijske sinteze neorganskih soli kod reakcija neutralizacije. Doktorska disertacija, Univerzitet u Beogradu; 2001.
Filipović S, Obradović N, Pavlović V, Marković S, Mitrić M, Mitrović N. Sinteza magnezijum titanata mehanohemijskom metodom. Tehnika. 2014; 23 (5): 727-731.