Lithium carbonate sedimentation using flocculants with different ionic bases Technical paper
Main Article Content
Abstract
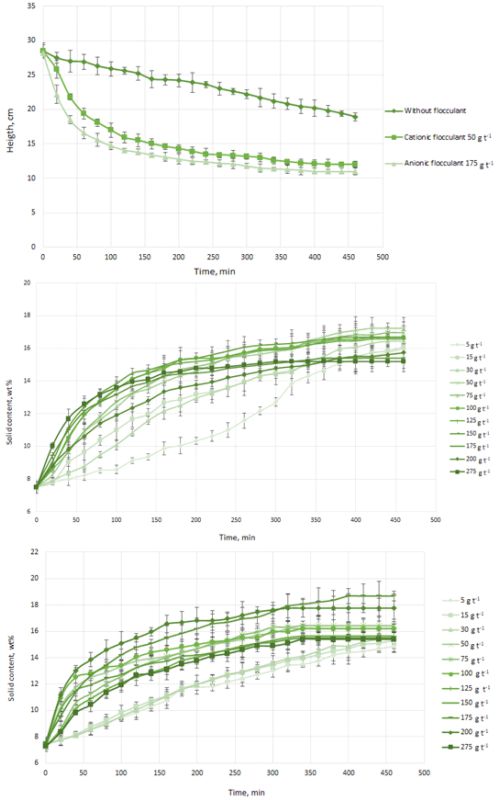
Lithium has become a metal of enormous interest worldwide. The extensive use of rechargeable batteries for a range of applications has pushed for rapid growth in demand for lithium carbonate. This compound is produced by crystallization, by reaction with lithium chloride (in solution) and by adding sodium carbonate. Low sedimentation rates in the evaporation pools present a problem in the crystallization process. For this reason, in this work, mineral sedimentation tests were carried out with the use of two flocculant types with different ionic charges. The tests were carried out at a laboratory level using different dosages for each flocculant and measurements were performed to obtain the increase in the content of solids in the sediment. The anionic flocculant had better performance as compared to that of the cationic flocculant, increasing the sedimentation rate of lithium carbonate by up to 6.5. However, similar solids contents were obtained with the use of the cationic flocculant at 3.5 times lower dosage making it the flocculant of choice regarding the economic point of view.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Saldaña M, Ayala L, Torres D, Toro N. Global sensitivity analyses of a neural networks model for a flotation circuit. Hemijska Industrija. 2020;74(4):247-256 https://doi.org/10.2298/HEMIND20060523S
Toro N, Briceño W, Pérez K, Cánovas M, Trigueros E, Sepúlveda R, Hernández P. Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water. Metals. 2019;9(7):780 https://doi.org/10.3390/met9070780
Toro N, Pérez K, Saldaña M, Jeldres RI, Jeldres M, Cánovas M. Dissolution of pure chalcopyrite with manganese nodules and waste water. Journal of Materials Research and Technology. 2019 https://doi.org/10.1016/j.jmrt.2019.11.020
Torres D, Pérez K, Trigueros E, Jeldres R, Salinas-Rodríguez E, Robles P, Toro N. Reducing-effect of chloride for the dissolution of black copper. Metals (Basel). 2020;10(1) https://doi.org/10.3390/met10010123
Pérez K, Toro N, Campos E, González J, Jeldres R, Nazer A, Rodriguez MH. Extraction of Mn from Black Copper Using Iron Oxides from Tailings and Fe2+ as Reducing Agents in Acid Medium. Metals (Basel). 2019;9:1112 https://doi.org/10.3390/met9101112
Toro N, Robles P, Jeldres R. Seabed mineral resources, an alternative for the future of renewable energy: A critical review. Ore Geology Reviews. August 2020:103699 https://doi.org/10.1016/j.oregeorev.2020.103699
Toro N, Jeldres R, Órdenes J, Robles P, Navarra A. Manganese Nodules in Chile , an Alternative for the Production of Co and Mn in the Future — A Review. Minerals. 2020;10(674):1-19 https://doi.org/10.3390/min10080674
Heelan J, Gratz E, Zheng Z, Wang Q, Chen M, Apelian D, Wang Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. Jom. 2016;68(10):2632-2638 http://doi.org/10.1007/s11837-016-1994-y
Maxwell P, Mora M. Lithium and Chile: looking back and looking forward. Mineral Economics. 2020;33(1-2):57-71 https://doi.org/10.1007/s13563-019-00181-8
Pinna EG, Drajlin DS, Toro N, Rodriguez MH. Kinetic modeling of the leaching of LiCoO2 with phosphoric acid. Journal of Materials Research and Technology. 2020;9(6):14017-14028 https://doi.org/10.1016/j.jmrt.2020.09.109
Donoso, F; Garay, V; Cantallopts J. International Lithium Market and Its Potential in Chile. Chile; 2017.
Gruber PW, Medina PA, Keoleian GA, Kesler SE, Everson MP, Wallington TJ. Global lithium availability: A constraint for electric vehicles? Journal of Industrial Ecology. 2011;15(5):760-775 https://doi.org/10.1111/j.1530-9290.2011.00359.x
U.S. Geological Survey. Mineral Commodity Summaries 2020.; 2020.
Velásquez C, Cabrera V. Small-scale lithium production from brines. In: Lima, Perú; 2018:19-21.
Lunde Seefeldt J. Lessons from the Lithium Triangle: Considering Policy Explanations for the Variation in Lithium Industry Development in the "Lithium Triangle" Countries of Chile, Argentina, and Bolivia. Politics and Policy. 2020;48(4):727-765.
Chen QB, Ji ZY, Liu J, Zhao YY, Wang SZ, Yuan JS. Development of recovering lithium from brines by selective-electrodialysis: Effect of coexisting cations on the migration of lithium. Journal of Membrane Science. 2018;548(8):408-420 https://doi.org/10.1016/j.memsci.2017.11.040
Miranda C. Background for a Public Policy in Strategic Minerals: Lithium. Chile; 2009.
Boryta, Kullberg T. Production of lithium compounds directly from lithium containing brines, in: Google Patents. 2007;2(12).
Jandová J, Dvořák P, Vu HN. Processing of zinnwaldite waste to obtain Li2CO3. Hydrometallurgy. 2010;103(1-4):12-18 https://doi.org/10.1016/j.hydromet.2010.02.010
Taborga P, Brito I, Graber TA. Effect of additives on size and shape of lithium carbonate crystals. Journal of Crystal Growth. 2017;460(November 2016):5-12 https://doi.org/10.1016/j.jcrysgro.2016.12.001
Han B, Anwar UI Haq R, Louhi-Kultanen M. Lithium carbonate precipitation by homogeneous and heterogeneous reactive crystallization. Hydrometallurgy. 2020;195(October 2019):105386 https://doi.org/10.1016/j.hydromet.2020.105386
Riveros Zapata A, Ale Ruiz L, Lezama J, Erdmann E. Lithium carbonate production: Simulation using aspen plus. Salta, Argentina; 2018.
Schoenmann, Hales, Bedell D. Strategies for instru-mentation and control of thickeners and other solid–liquid separation circuits. Mineral Processing Plant Design, Practice, and Control. 2002;2:2164-2173.
Teerikoski S. Optimal Control of Clarifier-Thickeners. Uppsala, Sweden; 2017 http://uu.diva-portal.org/smash/get/diva2:1088256/FULLTEXT01.pdf
Díaz J. Coagulants - organic and inorganic flocculants made from plants and scrap metal recycling, for the treatment of polluted water. McKinsey Quarterly. 2014;2(1):1-22.
Elhaei R, Kharrat R, Madani M. Stability, flocculation, and rheological behavior of silica suspension-augmented polyacrylamide and the possibility to improve polymer flooding functionality. Journal of Molecular Liquids. 2021;322:114572 https://doi.org/10.1016/j.molliq.2020.114572
Wilkomirsky I. Extraction and refining of non-ferrous metals: Lithium; Metallurgical engineering department, Universidad de Concepción, Concepción, Chile 2008:1-25.
Salam AM, Örmeci B, Simms PH. Determination of optimum polymer dosage for dewatering of oil sands tailings using torque rheology. Journal of Petroleum Science and Engineering. 2021;197 (August 2020) https://doi.org/10.1016/j.petrol.2020.107986
Concha F. Filtration and separation manual; Metallurgical engineering department, Universidad de Concepción, Concepción, Chile 2001;(January 2001):234-308.