Influence of suspension heating rate on properties of zeolite 13X Original scientific paper
Main Article Content
Abstract
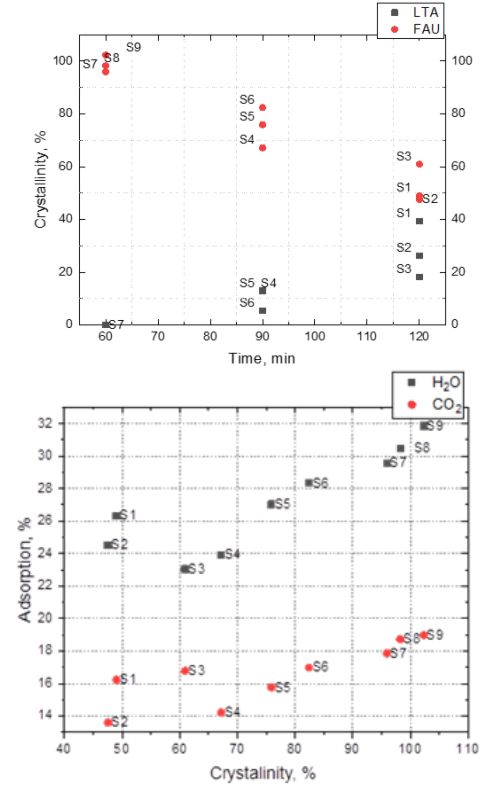
It is known that the temperature of crystallization during the synthesis of zeolite is one of the most important process parameters. However, during the research work on the synthesis of zeolite 13X and the introduction of this material into regular industrial production, it was noticed that the heating rate of the starting reaction suspension can have an equally important influence. This influence can be so pronounced that a difference of just a few minutes in reaching the crystallization temperature can make a significant difference in product quality, affect the presence of other phases in the crystal, or even determine the direction of zeolite crystallization. Therefore, the aim of this work was to show the influence of the heating rate on the quality of the obtained 13X zeolite powders. The obtained samples were analysed in terms of crystallinity (by X-ray diffraction), chemical composition, granulometry and specific surface area (by Brunauer-Emmett-Teller analysis), and regarding water and CO2 adsorption capacities. Additionally, scanning electron microscopy analysis of the samples showed the morphological characteristics of different 13X zeolite powders. The analysis results of the obtained powders confirmed the influence of the heating rate and helped to define the optimal synthesis parameters i.e. the initial temperature and heating time, that resulted in stable product quality.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Sameer SK, Eswaran P. Development and integration of oxygen generator for home air conditioner. IOP Conf Ser Mater Sci Eng 2020; 912: 042054 https://doi.org/10.1088/1757-899X/912/4/042054
Esfandian H, Garshasbi V. Investigation of methane adsorption on molecular sieve zeolite (from natural materials). Gas Process J 2020; 8(2): 35-50. https://doi.org/10.22108/gpj.2020.121907.1080
Brea P, Delgado JA, Águeda VI, Gutiérrez P, Uguina MA. Multicomponent adsorption of H2, CH4, CO and CO2 in zeolites NaX, CaX and MgX. Evaluation of performance in PSA cycles for hydrogen purification. Micropor Mesopor Mat 2019; 286: 187-198. https://doi.org/10.1016/J.MICROMESO.2019.05.021
Alshahidy BA, Abbas AS. Comparative study on the catalytic performance of a 13X zeolite and its dealuminated derivative for biodiesel production. Bull Chem React Eng Catal 2021; 16(4): 763-772. https://doi.org/10.9767/bcrec.16.4.11436.763-772
Senol S, Kaya B, Salt I, Tirnakci B, Salt Y. Pervaporation separation of ethylacetate-ethanol suspensions using zeolite 13X-filled poly(dimethylsiloxane) membrane. Chem Eng Commun 2022; 209 (8): 1048-1057. https://doi.org/10.1080/00986445.2021.1940155
Anbia M, Nejati FM, Jahangiri M, Eskandari A, Garshasbi V. Optimization of Synthesis Procedure for NaX Zeolite by Taguchi Experimental Design and its Application in CO2 Adsorption J Sci IRI. 2015; 26(3): 213–222 . https://jsciences.ut.ac.ir/article_55309.html
Ma Y, Yan C, Alshameri A, Qiu X, Zhou C, Li D. Synthesis and characterization of 13X zeolite from low-grade natural kaolin. Adv Powder Technol 2014; 25(2): 495-499. https://doi.org/10.1016/J.APT.2013.08.002
Zhang X, Tang D, Zhang M, Yang R. Synthesis of NaX zeolite: Influence of crystallization time, temperature and batch molar ratio SiO2/Al2O3 on the particulate properties of zeolite crystals. Powder Technol 2013; 235: 322-328. https://doi.org/10.1016/J.POWTEC.2012.10.046
Ngoc DT, Pham TH, Hong Nguyen KD. Synthesis, characterization and application of nanozeolite NaX from Vietnamese kaolin. Adv Nat Sci Nanosci Nanotechnol 2013; 4(4): 045018. https://doi.org/10.1088/2043-6262/4/4/045018
Prokof’ev VY, Gordina NE. Preparation of granulated LTA and SOD zeolites from mechanically activated suspensions of metakaolin and sodium hydroxide. Appl Clay Sci 2014; 101: 44-51. https://doi.org/10.1016/J.CLAY.2014.07.008
Sharma P, Song JS, Han MH, Cho CH. GIS-NaP1 zeolite microspheres as potential water adsorption material: Influence of initial silica concentration on adsorptive and physical/topological properties. Sci Rep 2016; 6: 22734. https://doi.org/10.1038/srep22734