Implementation of emergency measures to improve the efficiency of nickel removal from water at the existing water treatment plant Original scientific paper
Main Article Content
Abstract
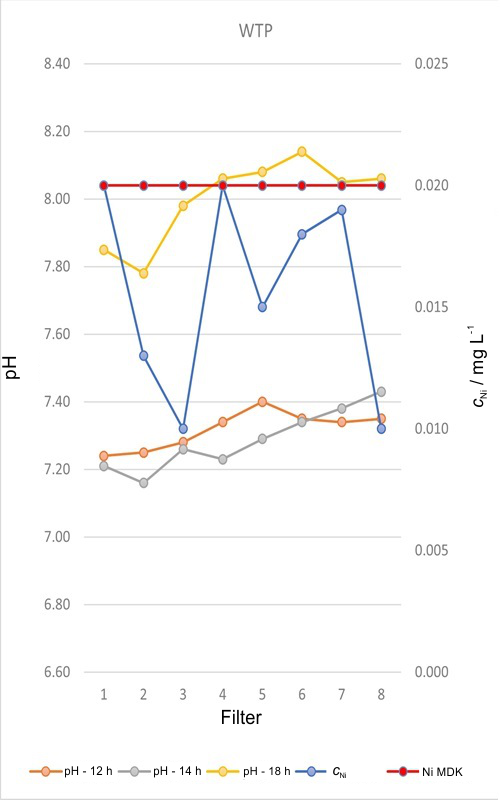
In this paper, based on the data of the Ribnica accumulation (Serbia), and the required quality of raw and treated water, an optimal solution for improving and optimization of the technology of water purification to drinking quality at a water treatment plant (WTP) in Zlatibor, Serbia, was proposed to ensure maximum efficiency and flexibility in system operation. Analysis of water quality has shown that after the treatment at the plant, all parameters were within the respective maximum available concentrations (MAC) stipulated by the Rulebook on the hygienic suitability of potable water of the Republic of Serbia, except for the nickel content. The paper presents the results of the nickel removal using multiple laboratory tests as well as at the WTP to achieve the best procedure for water treatment. In accordance with the results obtained the water quality problem in terms of the nickel removal was solved and the required effects are obtained (nickel content below the MAC, i.e. <0.02 mg dm-3)
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
[1] Rulebook on the hygienic suitability of drinking water. The Official Gazette of the FRY 42/98; 44/99; and Official Gazette of RS 28/19 (Pravilnik o ispravnosti vode za piće. Službeni glasnik RS 42/98; 44/99; 28/19; in Serbian) https://www.paragraf.rs/propisi/pravilnik-higijenskoj-ispravnosti-vode-pice.html
[2] Kutir P, College PG, Chakkey, J. Effect of pH on the removal of Ni (II). Int J Chem Sci. 2013; 11(3): 1493-1497. https://www.tsijournals.com/articles/effect-of-ph-on-the-removal-of-ni-ii.pdf
[3] Cruz-Lopes LP, Morgana M, Bruno E, Raquel PFG, Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 2021; 6:115-123. https://doi.org/10.1515/opag-2021-0225
[4] Hala AH. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013; 9(3):276-282. https://doi.org/10.1016/j.hbrcj.2013.08.004
[5] Utkarsh U, Sreedhar I. Singh SA, Patel CM, Anitha KL, Recent advances in heavy metal removal by chitosan based adsorbents Carbohydr Polym. 2021; 251: 117000 . https://doi.org/10.1016/j.carbpol.2020.117000
[6] Enshirah D. Adsorption of heavy metals on functionalized-mesoporous silica. Microporous Mesoporous Mater. 2017; 247: 145-157. https://doi.org/10.1016/j.micromeso.2017.03.050
[7] Rasheed T, Ahmad N., Nabeel F, Anwar MT, Bilal M. Metal-organic frameworks for removal of heavy metals. In Nano-Bioremediation: Fundamentals and Applications (pp. 455-476). Elsevier; 2021 https://doi.org/10.1016/B978-0-12-823962-9.00014-3
[8] Guo L, Biao Z, Jing L, Zeying W, Shixing W, Tu H, Libo Z. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem Eng J. 2023; 460: 141710. https://doi.org/10.1016/j.cej.2023.141710
[9] Eman AA, Reyad A. Al Dwairi ZS, Removal of nickel (II) ions from water by Jordan natural zeolite as sorbent material. J Saudi Chem Soc. 2021; 25: https://doi.org/10.1016/j.jscs.2021.101233
[10] Gopal R, Neelam C, Tapan A, Nickel as a Pollutant and its Management. Int Res J Environment Sci. 2014; 3(10): 94-98. https://www.isca.me/IJENS/Archive/v3/i10/15.ISCA-IRJEvS-2014-189.pdf
[11] Senthil KP, Ramakrishnan K, Gayathri R, Removal of nickel (II) from aqueous solutions by ceralite IR 120 cationic exchange resins. J Eng Sci Technol. 2010; 5(2): 232-243. https://jestec.taylors.edu.my/Vol%205%20Issue%202%20June%2010/Vol_5(2)_232_243_P%20SENTHIL%20KUMAR.pdf
[12] Оleg LD, Irina RV, Galina ZS, Konstantin MN, Inna BV, Yulia KV, Alexander GY, Boris GP, Zilara FA, Coagulation removal of nickel (II) ions by ferric chloride. Water Environ Res. 2022; 94: e10827. https://doi.org/10.1002/wer.10827
[13] Jaroslav Černi Water Institute, Conceptual Design for WTP Zlatibor, 2016. (Technical documentation in Serbian)
[14] Jaroslav Černi Water Institute, Design for Constriction Permit for WTP Zlatibor, 2016. (Technical documentation in Serbian)
[15] Zane Satterfield PE, Jar Testing. Tech Brief, Publisher by The National Environmental Services Center. 2005; Volume 5 (1). https://www.nesc.wvu.edu/files/d/3cf372e5-ba40-450c-a3ad-cd774f4c3345/jar-testing.pdf
[16] Nadem ZM, Nadeem R, Asif HM, Biosorption of nickel from protonated rice bran. J Hazard Mater. 2007; 143: 478–85. https://doi.org/10.1016/j.jhazmat.2006.09.055
[17] Dash RR, Balomajumder C, Kumar A. Removal of cyanide from water and wastewater using granular activated carbon. Chem Eng J. 2009; 146: 40-413. https://doi.org/10.1016/j.cej.2008.06.021
[18] Inc. Metcalf & Eddy, Tchobanoglou G, Stensel H, Tsuchihashi R, Burton F, Abu-Orf M, Bowden G, Pfrang W. Wastewater Engineering: Treatment and Resource Recovery. 5th ed., New York, NY, USA: McGraw-Hill Education; 2014: 634. https://www.amazon.com/Wastewater-Engineering-Treatment-Resource-Recovery/dp/0073401188
[19] Jaroslav Černi Water Institute, Preliminary Design for WTP Zlatibor, 2016. (Technical documentation, in Serbian)