Thermodynamic properties of binary mixtures of terpenes and 1-propanol in the temperature range from 288.15 to 323.15 K at atmospheric pressure Original scientific paper
Main Article Content
Abstract
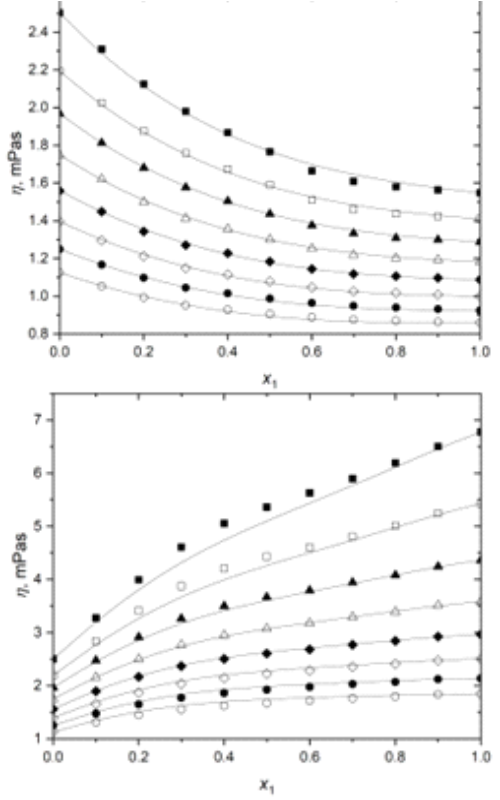
Terpenes are the most abundant class of chemical compounds present in essential oils. They are considered green solvents, and come from natural sources such as plants, citrus fruits, but also from tree leaves or pinecones. They find wide commercial uses in food industry as natural flavors and food additives, as well as in pharmaceutical and cosmetics industries. In order to study thermodynamic properties of binary mixtures of terpenes (α-pinene, p-cymene and linalool) with 1-propanol, density and viscosity of these mixtures were determined experimentally. Experimental measurements were done over the temperature range from 288.15 to 323.15 K at atmospheric pressure, over the entire composition range. Excess molar volumes, viscosity deviations and thermal expansion coefficients were calculated based on the experimental results of densities and viscosities. Experimentally measured properties were correlated using the Heritz-Brewer-Jouyban-Acree model, while the Redlich-Kister polynomial was used to correlate the derrived properties. All the experimentally obtained data and the derived values were used to analyze non-ideal behavior of the selected mixtures. The Heritz-Brewer-Jouyban-Acree model successfully correlated the experimental values for all three binary systems, while the Redlich-Kister successfully correlated the derived quantities.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministry of Scientific and Technological Development, Higher Education and Information Society,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-65/2024-03/200135
References
[1] Jiang Z, Kempinski C, Chappell J, Extraction and analysis of terpenes/terpenoids. Curr Protoc Plant Biol. 2006; 1(2): 345-358. https://doi.org/10.1002/cppb.20024
[2] Rodriguez-Garcia A, Hosseini S, Martinez-Chapa S, Cordell A, Multi-target activities of selected alkaloids and terpenoids. Mini-Rev Org Chem. 2017; 14(4): 272-279. 10.2174/1570193X14666170518151027
[3] Papada E, Gioxari A, Brieudes V, Amerikanou C, Halabalaki M, Skaltsounis L, Kaliora C. Bioavailability of terpenes and postprandial effect on human antioxidant potential. An open‐label study in healthy subjects. Mol Nut & Food Res. 2018; 62(3): 1700751. https://doi.org/10.1002/mnfr.201700751
[4] Allenspach M, Steuer C. α-Pinene: A never-ending story. Phytochem. 2021; 190: 112857. https://doi.org/10.1016/j.phytochem.2021.112857
[5] Jung K, Lee Y, Choi W, Jae J, Ha M, Suh J, Lee Y. Production of high-energy-density fuels by catalytic β-pinene dimerization: effects of the catalyst surface acidity and pore width on selective dimer production. Energy Conv and Manag. 2016; 116: 72-79. http://dx.doi.org/10.1016/j.enconman.2016.02.053
[6] Oswald P, Whitside R, Schäffer J, Köhler M. An experimental flow reactor study of the combustion kinetics of terpenoid jet fuel compounds: Farnesane, p-menthane and p-cymene. Fuel. 2017; 187: 43-50. http://dx.doi.org/10.1016/j.fuel.2016.09.035
[7] Balahbib A, El Omari N, Hachlafi E, Lakhdar F, El Menyiy N, Salhi N, Bouyahya A. Health beneficial and pharmacological properties of p-cymene. Food and Chem Tox. 2021; 153: 112259. https://doi.org/10.1016/j.fct.2021.112259
[8] Marchese A, Arciola R, Barbieri R, Silva S, Nabavi F, Tsetegho Sokeng J., Nabavi M. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials. 2017; 10(8): 947. https://doi.org/10.3390/ma10080947
[9] Cheng H, Lin Y, Yeh F, Cheng S, Chang T. Potential source of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaf: essential oil profile and enantiomeric purity. J Agric & Food Chem. 2012; 60(31): 7623-7628. https://doi.org/10.1021/jf302248w
[10] Hussain I, Anwar F, Sherazi H, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008; 108(3): 986-995. https://doi.org/10.1016/j.foodchem.2007.12.010
[11] Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food & Chem Toxic. 2008; 46(2): 446-475. https://doi.org/10.1016/j.fct.2007.09.106
[12] Meylemans A, Quintana L, Goldsmith R, Harvey G. Solvent‐free conversion of linalool to methylcyclopentadiene dimers: a route to renewable high‐density fuels. Chem Sus Chem. 2011; 4(4): 465-469. https://doi.org/10.1002/cssc.201100017
[13] Radovic I, Grozdanic N, Djordjevic B, Serbanovic S, Kijevcanin M. Prediction of excess molar volumes of binary mixtures by Prigogine-Flory-Patterson (PFP) and extended real association solution (ERAS) models, J Serb Chem Soc. 2017; 82: 1379-1390. http://dx.doi.org/10.2298/JSC170817103R
[14] Grozdanic N, Radovic I, Knezevic-Stevanovic A, Kijevcanin M. Volumetric properties of binary mixtures of tetrahydrofuran, dimethyl adipate, 1-butanol and 2-butanol from (288.15 to 323.15) K and modeling by Prigogine-Flory-Patterson (PFP) and Extended Real Association Solution (ERAS) models. J Mol Liq. 2021; 340: 117313. https://doi.org/10.1016/j.molliq.2021.117313
[15] Ilic-Pajic J, Radovic I, Grozdanic N, Stajic-Trosic J, Kijevcanin M. Volumetric and thermodynamic properties of binary mixtures of p-cymene with α-pinene, limonene and citral at atmospheric pressure and temperatures up to 323.15 K. Journal of Molecular Liquids. 2021; 344: 117486. https://doi.org/10.1016/j.molliq.2021.117486
[16] Ribeiro A, Bernardo-Gil G. Densities and refractive indices of components of pine resin. J Chem Eng Data. 1990; 35: 204-206. https://doi.org/10.1021/je00060a033
[17] Liao D-K, Meng X-L, Tong Z-F, Zheng D-X, Peng D-Y, Lu B. Excess Molar Enthalpies of p-cymene + α-Pinene + β-Pinene at (298.15, 308.15 and 318.15) K and at Atmospheric Pressure. J Chem Eng Data. 2007; 52: 808-811. http://dx.doi.org/10.1021/je060420p
[18] Clara R, Gomez Marigliano A, Solimo H. Density, Viscosity, and Refractive Index in the Range (283.15 to 353.15) K and Vapor Pressure of r-Pinene, d-Limonene, (()-Linalool, and Citral Over the Pressure Range 1.0 kPa Atmospheric Pressure. J Chem Eng Data. 2009; 54: 1087–1090. http://dx.doi.org/10.1021/je8007414
[19] Florido M, Andrade G, Capellini C, Carvalho H, Aracava K, Koshima C, Rodrigues C, Goncalves B. Viscosities and densities of systems involved in the deterpenation of essential oils by liquid-liquid extraction: New UNIFAC-VISCO parameters. J Chem Thermodyn. 2014; 72: 152-160. https://doi.org/10.1016/j.jct.2013.11.026
[20] Comelli F, Francesconi R, Castellari C. Densities, Viscosities, and Excess Molar Enthalpies of Binary Mixtures Containing Essential Oils at (298.15 and 313.15) K. The (S)-(−)-Limonene + Cineole, (S)-(−)-Limonene + Linalool, and Cineole + Linalool Systems. J Chem Eng Data. 2001; 46: 868–872. https://doi.org/10.1021/je010005r
[21] Comelli F, Ottani S, Francesconi R, Castellari C. Densities, Viscosities, and Refractive Indices of Binary Mixtures Containing n-Hexane + Components of Pine resins and Essential Oils at 298.15 K. J Chem Eng Data. 2002; 47: 93-97. https://doi.org/10.1021/je010216w
[22] Vercher E, Orchilles V, Miguel P, Martinez-Andreu A. Volumetric and Ultrasonic Studies of 1-Ethyl-3-methylimidazolium Trifluoromethanesulfonate Ionic Liquid with Methanol, Ethanol, 1-Propanol, and Water at Several Temperatures. J Chem Eng Data. 2007; 52: 1468-1482. https://doi.org/10.1021/je7001804
[23] Pal A, Kumar A. Viscosity of 1-Propanol + Ethylene Glycol Dimethyl, + Diethylene Glycol Dimethyl, + Triethylene Glycol Dimethyl, and + Tetraethylene Glycol Dimethyl Ethers at 288.15, 298.15 and 308.15 K. Ind J Chem A. 2003; 42: 2708-2716. https://nopr.niscpr.res.in/bitstream/123456789/20780/1/IJCA%2042A(11)%202708-2716.pdf
[24] Yang C, Lai H, Liu Z, Ma P. Density and Viscosity of Binary Mixtures of Diethyl Carbonate with Alcohols at (293.15 to 363.15) K and Predictive Results by UNIFAC-VISCO Group Contribution Method. J Chem Eng Data. 2006; 51: 1345-1351. http://dx.doi.org/10.1021/je0600808
[25] Rodriguez A, Canosa J, Dominguez A, Tojo J. Dynamic viscosities of diethyl carbonate with linear and secondary alcohols at several temperatures. J Chem Eng Data. 2004; 49: 157-162. http://dx.doi.org/10.1021/je0341413
[26] Saleh A, Habibullah M, Ahmed S, Uddin A, Uddin H, Khan M, Excess Molar Volumes and Viscosities of Some Alkanols with Cumene. Phys Chem Liq. 2006; 44: 31-43. http://dx.doi.org/10.1080/00319100500287853
[27] Jouyban A, Khoubnasabjafari M, Vaez-Gharamaleki Z, Fekari Z, Eugene Jr W. Calculation of the viscosity of binary liquids at various temperatures using Jouyban–Acree model. Chem and Pharm Bull. 2005; 53(5): 519-523. https://doi.org/10.1248/cpb.53.519
[28] Wan Normazlan D, Sairi A, Alias Y, Udaiyappan F, Jouyban A, Khoubnasabjafari M. Composition and temperature dependence of density, surface tension, and viscosity of EMIM DEP/MMIM DMP+ water+ 1-propanol/2-propanol ternary mixtures and their mathematical representation using the Jouyban–acree model. J Chem Eng Data. 2014; 59(8): 2337-2348. http://dx.doi.org/10.1021/je400576e
[29] Redlich O, Kister T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind & Eng Chem. 1948; 40(2): 345-348. https://doi.org/10.1021/ie50458a036