Fenton-like oxidative degradation of Orange G dye and binary dye mixtures using Oxone® activated with cobalt-doped alumina catalysts Original scientific paper
Main Article Content
Abstract
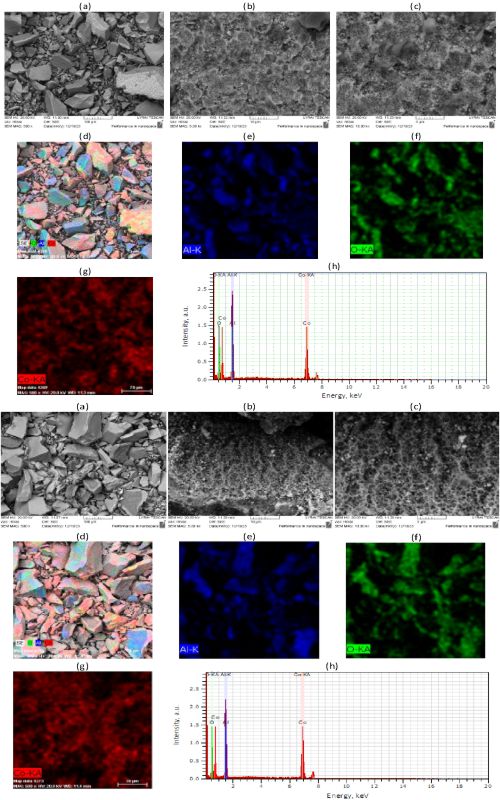
Two texturally and structurally different Co-doped aluminas were obtained by using the sol-gel method followed by calcination at temperatures of 1000 °C and 1100 °C. The obtained materials were tested as catalysts in anionic textile dye Orange G (OG) degradation using Oxone® as a precursor of sulfate anion radicals, the main reactive oxygen species. Effects of temperature and initial pH on degradation efficiency was investigated. The increase in temperature accelerated the reaction rate and the maximal degradation efficiency was obtained at 60 °C. Different kinetic models were applied and pseudo-first order rate was found to be the most appropriate. Both catalysts showed the optimal performance in the pH range around neutral. Coexisting cations (Ca2+, Mg2+, K+ and Na+) enhanced the OG degradation rate, as well as anions: Clˉ and H2PO4ˉ, while NO3ˉ, SO42ˉand HCO3ˉ inhibited the degradation. The catalysts were also proved effective in degradation of the other investigated dyes: Methylene blue, Basic blue 41, and Remazol brilliant blue R. Finally, simultaneous degradation of OG in binary dye mixtures was investigated showing that the synthesized catalysts can be also used in simultaneous processes of dye degradation. However, differences in structural and textural properties of the two catalysts affected their catalytic performance.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200026
References
[1] Busca G. Structural, surface, and catalytic properties of aluminas. In: Jentoft F, ed. Advances in Catalysis, Norman, Oklahoma, USA, Elsevier Inc. 2014; 57: 2-412, ISSN 0360-0564; http://dx.doi.org/10.1016/B978-0-12-800127-1.00003-5
[2] Maldonado CS, De la Rosa JR, Lucio-Ortiz C, Hernández-Ramírez A, Castillón Barraza F, Valente J. Low concentration Fe-doped alumina catalysts using sol-gel and impregnation methods: The synthesis, characterization and catalytic performance during the combustion of trichloroethylene. Materials. 2014; 7: 2062-2086. http://dx.doi.org/10.3390/ma7032062
[3] Jiratova K, Beranek L, Properties of modified aluminas. Appl Catal. 1982; 2: 125-138. http://doi.org/10.1016/0166-9834(82)80196-6
[4] Matori KA, Wah LC, Hashim M, Ismail I, Mohd Zaid MH. Phase transformations of α-alumina made from waste aluminum via a precipitation technique., Int J Mol Sci. 2012; 13: 16812-16821 doi:10.3390/ijms131216812
[5] Gitzen WH. Alumina as a ceramic material; Wiley-American Ceramic Society, Columbus, OH, USA, 1970.
[6] Khattak AK, Afzal M, Saleem M, Yasmeen G, Ahmad R, Surface modifcation of alumina by metal doping, Colloid surf A 2000; 162: 99-106 https://doi.org/10.1016/S0927-7757(99)00218-6.
[7] Marinović S, Mudrinić T, Dojčinović B, Barudžija T, Banković P, Novaković T, Cobalt-doped alumina catalysts in catalytic oxidation of tartrazine induced by Oxone®. J Environ Chem Eng. 2021; 9: 106348 (8 pages). https://doi.org/10.1016/j.jece.2021.106348
[8] Jovanović A, Bugarčić M, Sokić M, Barudžija T, Pavićević V, Marinković A, Photodegradation of thiophanate-methyl under simulated sunlight by utilization of novel composite photocatalysts. Hem Ind. 2024. https://doi.org/10.2298/HEMIND230523004J
[9] Hu P, Long M, Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl Catal. 2016; 181: 103-117.http://dx.doi.org/10.1016/j.apcatb.2015.07.024
[10] Nfodzo P, Choi H, Triclosan decomposition by sulfate radicals: Effects of oxidant and metal doses. Chem Eng J. 2011; 174: 629-634.http://dx.doi.org/10.1016/j.cej.2011.09.076
[11] Zhou ZG, Du HM, Dai Z, Mu Y, Tong LL, Xing QJ, Liu SS, Ao Z, Zou JP, Degradation of organic pollutants by peroxymonosulfate activated by MnO2 with different crystalline structures: Catalytic performances and mechanisms. Chem Eng J. 2019; 374: 170-180. https://doi.org/10.1016/j.cej.2019.05.170
[12] Stevanović G, Jović-Jovičić N, Popović A, Dojčinović B, Milutinović-Nikolić A, Banković P, Ajduković M, Degradation of textile dyes by Oxone® activated by cobalt supported chitosan-derived carbon-smectite catalyst. Sci Sinter. 2024; https://doi.org/10.2298/SOS230427037S
[13] KulićMandić A, Bečelić Tomin M, PucarMilidrag G, Rašeta M, Kerkez Đ, Application of impregnated biocarbon produced from soybean hulls in dye decolorization. Hem Ind. 2021; 75: 307-320. https://doi.org/10.2298/HEMIND210427023K
[14] Ganesan S, Kokulnathan T, Sumathi S, Palaniappan A, Efficient photocatalytic degradation of textile dye pollutants using thermally exfoliated graphitic carbon nitride (TE-g-C3N4). Sci Rep. 2024; 14: 2284. https://doi.org/10.1038/s41598-024-52688-y
[15] Far HS, Hasanzadeh M, Najafi M, Rabbani M, Highly porous organoclay-supported bimetal-organic framework (CoNi-MOF/OC) composite with efficient and selective adsorption of organic dyes. Environ Sci Pollut Res. 2023; 30: 43714-43725
[16] https://doi.org/10.1007/s11356-023-25374-1
[17] Thao LT, Nguyen TV, Nguyen VQ, Phan NM, Ki Jae Kim, Huy NN, Dung NT, Orange G degradation by heterogeneous peroxymonosulfate activation based on magnetic MnFe2O4/α-MnO2 hybrid, J Environ Sci. 2023; 124: 379-396 https://doi.org/10.1016/j.jes.2021.10.008.
[18] Zhang J, Zhua MCL, Activation of peroxymonosulfate by iron-based catalysts for orange G degradation: role of hydroxylamine. RSC Adv. 2016; 6: 47562-47569 https://doi.org/10.1039/C6RA07231C
[19] Wu M, Wang Y, Lu B, Xiao B, Chen R, Liu H, Efficient activation of peroxymonosulfate and degradation of Orange G in iron phosphide prepared by pickling waste liquor. Chemosphere. 2021; 269: 129398.https://doi.org/10.1016/j.chemosphere.2020.129398
[20] Yu B, Li Z, Zhang S, Zero-valent copper-mediated peroxymonosulfate activation for efficient degradation of azo dye Orange G. Catalysts. 2022; 12: 700. https://doi.org/10.3390/catal12070700
[21] Madihi-Bidgoli S, Asghari F, Cheraghi S, Hamidinia H, Shagerdi E, AsadnezhadS, UV/periodate and UV/chlorine for dye degradation and real wastewater treatment: a comparative study, Water Pract Technol. 2023; 18: 2453-2468.https://doi.org/10.2166/wpt.2023.160
[22] Li C, Huang Y, Dong X, Sun Z, Duan X, Ren B, Zheng S, Dionysiou DD, Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine, ApplCatal B: Environ. 2019; 247: 10-23. https://doi.org/10.1016/j.apcatb.2019.01.079
[23] Zhou G, Xu Y, Zhang X, Sun Y, Wang C, Yu P, Efficient Activation of Peroxymonosulfate by Cobalt Supported Used Resin Based Carbon Ball Catalyst for the Degradation of Ibuprofen. Materials. 2022; 15:5003. https://doi.org/10.3390/ma15145003
[24] Li N, Wang Y, Cheng X, Dai H, Yan B, Chen G, Hou L, Wang S, Influences and mechanisms of phosphate ions onto persulfate activation and organic degradation in water treatment: A review, Water Res. 2022; 222: 118896. https://doi.org/10.1016/j.watres.2022.118896
[25] Sheng B, Huang Y, Wang Z, Yang F, AiL, Liu J,On peroxymonosulfate-based treatment of saline wastewater: When phosphate and chloride co-exist,RSC Adv.2018; 8: 13865. http://doi.org/10.1039/c8ra00600h
[26] Zhao X, Ana QD, Xiao ZY, Zhai SR, Shi Z, Seaweed-derived multifunctional nitrogen/cobalt-co doped carbonaceous beads for relatively high-efficient peroxymonosulfate activation for organic pollutants degradation, Chem Eng J. 2018; 353: 746-759. https://doi.org/10.1016/j.cej.2018.07.171
[27] Yuan R, Ramjaun SN, Wang Z, Liu J, Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds, J Hazard Mater. 2011; 196: 173-179. https://doi.org/10.1016/j.jhazmat.2011.09.007
[28] Xu A, Wei Y, Zou Q, Zhang W, Jin Y, Wang Z, Yang L, Li X, The effects of nonredox metal ions on the activation of peroxymonosulfate for organic pollutants degradation in aqueous solution with cobalt based catalysts: A new mechanism investigation, J Hazard Mater. 2020; 382: 121081. https://doi.org/10.1016/j.jhazmat.2019.121081
[29] Lončarević D, Dostanić J, Radonjić V, ŽivkovićLj, Jovanović D, Simultaneous photodegradation of two textile dyes usingTiO2 as a catalyst, React Kinet Mech Cat. 2016; 118: 153-164. http://doi.org/ 10.1007/s11144-016-0990-0
[30] Lin KYA, Lin TY, Degradation of Acid Azo Dyes Using Oxone Activated by Cobalt Titanate Perovskite, Water Air Soil Pollut. 2018; 229:10. https://doi.org/10.1007/s11270-017-3648-2
[31] Verma S, Rao BT, Singh R, Kaul R, Photocatalytic degradation kinetics of cationic and anionic dyes using Au-ZnO nanorods: Role of pH for selective and simultaneous degradation of binary dye mixtures, Ceram Int. 2021; 47: 34751-34764.https://doi.org/10.1016/j.ceramint.2021.09.014