Multi-walled carbon nanotubes as lipase carriers for organic synthesis: current trends and recent update Review paper
Main Article Content
Abstract
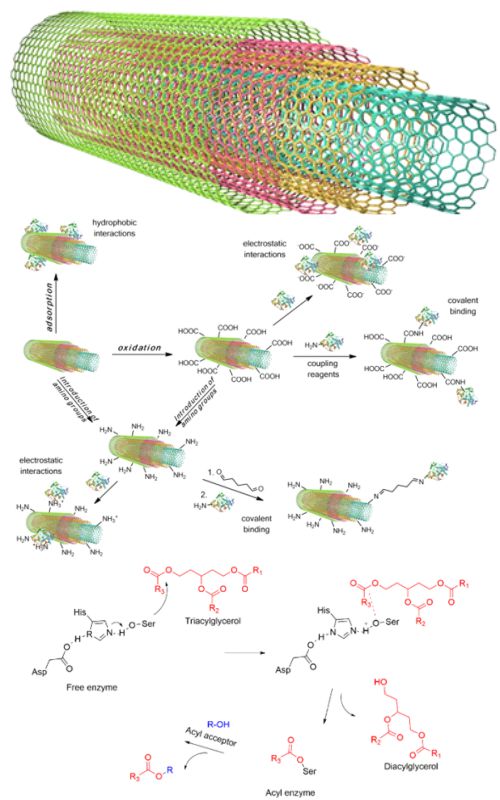
Lipase-catalyzed organic reactions have been widely practiced in the past three decades. Especially interesting are insoluble/immobilized forms due to providing a possibility of facile use and recyclability, thus reducing process costs, and making the procedure more environmentally friendly. Carbon-based supports have been extensively exploited for this purpose, because of neutral and biodegradable nature and thermal and chemical stability. Their high specific surface area, characteristic surface morphology and lower mass transfer resistances play a vital role in the performance of the attached enzyme. This review paper presents an overview of the main aspects of lipase immobilized on multi-walled carbon nanotubes (MWCNTs). Moreover, different immobilization strategies to achieve a biocatalyst with improved performances are discussed. Furthermore, as lipases are considered to have high commercial worth for synthesis of valuable organic molecules, the second part of the paper is dedicated to the overview of the most important industrial sectors in which these nanobiocatalysts have been used. In specific, applications in biodiesel production, flavour ester synthesis and racemization are summarized.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200135
References
Winkler CK, Schrittwieser JH, Kroutil W. Power of Biocatalysis for Organic Synthesis. ACS Cent Sci. 2021; 7(1): 55-71. https://doi.org/10.1021/acscentsci.0c01496
Sutradhar M. Metal-based catalysts in organic synthesis. Catalysts. 2020; 10(12): 1429. https://doi.org/10.3390/catal10121429
Cossy J. Biocatalyts: Catalysts of the future for organic synthesis and beyond? Tetrahedron. 2022; 123: 132966. https://doi.org/10.1016/j.tet.2022.132966
Sheldon RA, Woodley JM. Role of Biocatalysis in Sustainable Chemistry. Chem Rev. 2018; 118(2): 801-838. https://doi.org/10.1021/acs.chemrev.7b00203
Heckmann CM, Paradisi F. Looking Back: A Short History of the Discovery of Enzymes and How They Became Powerful Chemical Tools. ChemCatChem. 2020; 12(24): 6082-6102. https://doi.org/10.1002/cctc.202001107
Koeller KM, Wong CH. Enzymes for chemical synthesis. Nature. 2001; 409(6817): 232-240. https://doi.org/10.1038/35051706
Guisan JM, Fernandez-Lorente G, Rocha-Martin J, Moreno-Gamero D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr Opin Green Sustain Chem. 2022; 35: 100593. https://doi.org/10.1016/j.cogsc.2022.100593
Gutarra MLE, Miranda LSM, de Souza ROMA. Enzyme Immobilization for Organic Synthesis. In: Goswami A, Stewart JD, eds. Organic Synthesis Using Biocatalysts. Academic Press; Elsevier Inc.; 2016: 99-126. https://doi.org/10.1016/B978-0-12-411518-7.00004-4
Padrosa DR, Benítez-Mateos AI, Paradisi F. Back to the future: taking enzymes to the next level of sustainability. Biochem (Lond). 2022; 44(3): 19-22. https://doi.org/10.1042/bio_2022_109
Kazlauskas R. Hydrolysis and Formation of Carboxylic Acid and Alcohol Derivatives. In: Goswami A, Stewart JD, eds. Organic Synthesis Using Biocatalysts. Academic Press; Elsevier; 2016: 127-148. https://doi.org/10.1016/B978-0-12-411518-7.00005-6
Prlainović NŽ., Bezbradica DI., Knežević-Jugović ZD., Kozlowska RT., Mijin D Ž. A Kinetic Study of Candida rugosa Lipase-Catalyzed Synthesis of 4,6-Dimethyl-3-cyano-2-pyridone. J Brazilian Chem Soc. 2010; 21(12): 2285-2293. https://doi.org/10.1016/0096-3003(83)90011-5
Prlainović NŽ, Bezbradica DI, Knežević-Jugović ZD, Marinković AD, Mijin DŽ. Imobilizacija enzima na ugljenične nanocevi. Hem Ind. 2011; 65(4): 423-430. https://doi.org/10.2298/HEMIND110330028P (in Serbian)
Prlainović NŽ, Bezbradica DI, Knežević-Jugović ZD, Veličković D V., Mijin DŽ. Enzymatic synthesis of a vitamin B6 precursor. J Serb Chem Soc. 2013; 78(10): 1491-1501. https://doi.org/10.2298/JSC130322050P
Milašinović N, Jakovetić S, Knežević-Jugović Z, Milosavljević N, Lučić M, Filipović J, Kalagasidis Krušić M. Catalyzed ester synthesis using Candida rugosa lipase entrapped by poly(N-isopropylacrylamide-co-itaconic Acid) hydrogel. Sci World J. 2014; 2014: 142123. https://doi.org/10.1155/2014/142123
Milašinović N, Knežević-Jugović Z, Jakovljević Ž, Filipović J, Kalagasidis Krušić M. Synthesis of n-amyl isobutyrate catalyzed by Candida rugosa lipase immobilized into poly(N-isopropylacrylamide-co-itaconic acid) hydrogels. Chem Eng J. 2012; 181-182: 614-623. https://doi.org/10.1016/J.CEJ.2011.11.115
Dwivedee BP, Soni S, Sharma M, Bhaumik J, Laha JK, Banerjee UC. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect. 2018; 3(9): 2441-2466. https://doi.org/10.1002/slct.201702954
Chibata I, Tosa T. Immobilized Cells: Historical Background. Appl Biochem Bioeng. 1983; 4: 1-9. https://doi.org/10.1016/b978-0-12-041104-7.50007-5
Homaei AA, Sariri R, Vianello F, Stevanato R. Enzyme immobilization: An update. J Chem Biol. 2013; 6(4): 185-205. https://doi.org/10.1007/s12154-013-0102-9
Cao L. Introduction: Immobilized Enzymes: Past, Present and Prospects. In: Carrier-bound Immobilized Enzymes, Wiley; 2005; 1-52. https://doi.org/10.1002/3527607668.ch1
Zdarta J, Meyer AS, Jesionowski T, Pinelo M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts. 2018; 8(2): 92. https://doi.org/10.3390/catal8020092
Spasojević M, Prodanović O, Pantić N, Popović N, Balaž AM, Prodanović R. The Enzyme Immobilization: Carriers and Immobilization methods. J Eng Process Manag. 2019; 11(2): 89-105. https://doi.org/10.7251/jepm1902089s
Ismail AR, Baek KH. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int J Biol Macromol. 2020; 163: 1624-1639. https://doi.org/10.1016/j.ijbiomac.2020.09.021
Mihailović M, Stojanović M, Banjanac K, Carević M, Prlainović N, Milosavić N, Bezbradica D. Immobilization of lipase on epoxy-activated Purolite® A109 and its post-immobilization stabilization. Process Biochem. 2014; 49(4): 637-646. https://doi.org/10.1016/j.procbio.2014.01.013
Prlainović NŽ, Knežević-Jugović ZD, Mijin DŽ, Bezbradica DI. Immobilization of lipase from Candida rugosa on Sepabeads®: The effect of lipase oxidation by periodates. Bioprocess Biosyst Eng. 2011; 34(7): 803-810. https://doi.org/10.1007/s00449-011-0530-2
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018; 9(1): 1050-1074. https://doi.org/10.3762/bjnano.9.98
Veličić Z, Rusmirović J, Prlainović N, Tomić N, Veličković Z, Taleb K, Marinković AD. The optimization of glycidyl methacrylate based terpolymer monolith synthesis: an effective Candida rugosa lipase immobilization support. J Polym Res. 2020; 27(5): 127. https://doi.org/10.1007/s10965-020-02127-z
Buzea C, Pacheco I. Nanomaterials and their classification. Adv Struct Mater. 2017; 62: 3-45. https://doi.org/10.1007/978-81-322-3655-9_1
Meryam Sardar RA. Enzyme Immobilization: An Overview on Nanoparticles as Immobilization Matrix. Biochem Anal Biochem. 2015; 4(2): 1000178. https://doi.org/10.4172/2161-1009.1000178
Banjanac K, Carević M, Ćorović M, Milivojević A, Prlainović N, Marinković A, Bezbradica D. Novel β-galactosidase nanobiocatalyst systems for application in the synthesis of bioactive galactosides. RSC Adv. 2016; 6(99): 97216-97225. https://doi.org/10.1039/c6ra20409k
Rao N, Singh R, Bashambu L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater Today Proc. 2021; 44: 608-614. https://doi.org/10.1016/j.matpr.2020.10.593
Romero-Arcos M, Pérez-Robles JF, Guadalupe Garnica-Romo M, Luna-Martinez MS, Gonzalez-Reyna MA. Synthesis and functionalization of carbon nanotubes and nanospheres as a support for the immobilization of an enzyme extract from the mushroom Trametes versicolor. J Mater Sci. 2019; 54(17): 11671-11681. https://doi.org/10.1007/s10853-019-03722-2
Iijima S. Helical microtubules of graphitic carbon. Nature. 1991; 354: 56-58. https://doi.org/10.1038/354056a0
Khan M, Husain Q. Multiwalled carbon nanotubes bound beta-galactosidase: It’s activity, stability and reusability. Methods Enzymol. 2020; 630: 365-405. https://doi.org/10.1016/bs.mie.2019.10.018
Taib NAB, Uddin J, Khusairy M, Bakri B. A Review on Carbon Nanotubes (CNT): Structure, Synthesis, Purification and Properties for Modern day Applications. Res Sq. 2021: 1-22. https://doi.org/10.21203/rs.3.rs-930166/v1
Bilal M, Anh Nguyen T, Iqbal HMN. Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization - A paradigm shift in biocatalyst design. Coord Chem Rev. 2020; 422: 213475. https://doi.org/10.1016/j.ccr.2020.213475
Shuai W, Kumar Das R, Naghdi M, Kaur Brar S, Verma M. A Review on the ImportantAspects of Lipase Immobilization on Nanomaterials. Biotechnol Appl Biochem Appl Biochem. 2017; 64(4): 496-508. https://doi.org/10.1002/bab.1515
Mokhtar NF, Raja Noor Zaliha RNZR, Muhd Noor ND, Mohd Shariff F, Ali MSM. The immobilization of lipases on porous support by adsorption and hydrophobic interaction method. Catalysts. 2020; 10(7): 1-17. https://doi.org/10.3390/catal10070744
Zniszczoł A, Herman AP, Szymańska K, Mrowiec-Białoń J, Walczak KZ, Jarzebski A, Boncel S. Covalently immobilized lipase on aminoalkyl-, carboxy- and hydroxy-multi-wall carbon nanotubes in the enantioselective synthesis of Solketal esters. Enzyme Microb Technol. 2016; 87-88: 61-69. https://doi.org/10.1016/j.enzmictec.2016.02.015
Pavlidis IV, Tsoufis T, Enotiadis A, Gournis D, Stamatis H. Functionalized multi-wall carbon nanotubes for lipase immobilization. Adv Eng Mater. 2010; 12(5): 179-183. https://doi.org/10.1002/adem.200980021
Vrutika P, Datta M. Lipase from Solvent-Tolerant Pseudomonas sp. DMVR46 Strain Adsorb on Multiwalled Carbon Nanotubes: Application for Enzymatic Biotransformation in Organic Solvents. Appl Biochem Biotechnol. 2015; 177(6): 1313-1326. https://doi.org/10.1007/s12010-015-1816-7
Prlainović NŽ, Bezbradica DI, Knežević-Jugović ZD, Stevanović SI, Avramov Ivić ML, Uskoković PS, Mijin DŽ. Adsorption of lipase from Candida rugosa on multi walled carbon nanotubes. J Ind Eng Chem. 2013; 19(1): 279-285. https://doi.org/10.1016/j.jiec.2012.08.012
Markiton M, Boncel S, Janas D, Chrobok A. Highly active nanobiocatalyst from lipase noncovalently immobilized on multiwalled carbon nanotubes for Baeyer-Villiger synthesis of lactones. ACS Sustain Chem Eng. 2017; 5(2): 1685-1691. https://doi.org/10.1021/acssuschemeng.6b02433
Szelwicka A, Boncel S, Jurczyk S, Chrobok A. Exceptionally active and reusable nanobiocatalyst comprising lipase non-covalently immobilized on multi-wall carbon nanotubes for the synthesis of diester plasticizers. Appl Catal A Gen. 2019; 574: 41-47. https://doi.org/10.1016/j.apcata.2019.01.030
Ghide MK, Yan Y. 1,3-Dioleoyl-2-palmitoyl glycerol (OPO)—Enzymatic synthesis and use as an important supplement in infant formulas. J Food Biochem. 2021; 45(7): 13799. https://doi.org/10.1111/jfbc.13799
Szelwicka A, Siewniak A, Kolanowska A, Boncel S. Selective Synthesis of Levulinate Esters. Materials (Basel). 2021; 14: 1518. https://doi.org/10.3390/ma14061518
Szelwicka A, Kolanowska A, Latos P, Jurczyk S, Boncel S, Chrobok A. Carbon nanotube/PTFE as a hybrid platform for lipase B from Candida antarcticain transformation of α-angelica lactone into alkyl levulinates. Catal Sci Technol. 2020; 10(10): 3255-3264. https://doi.org/10.1039/d0cy00545b
Ameri A, Forootanfar H, Behnam B, Shakibaie M, Ameri A, Daneshpajooh M, Najafi A, Amirheidari B. Optimization of immobilization of Pseudomonas cepacia lipase on multiwalled carbon nanotubes functionalized with glycyrrhizin and Tween 80. 3 Biotech. 2021; 11(6): 1-13. https://doi.org/10.1007/s13205-021-02813-9
Che Marzuki NH, Mahat NA, Huyop F, Aboul-Enein HY, Wahab RA. Sustainable production of the emulsifier methyl oleate by Candida rugosa lipase nanoconjugates. Food Bioprod Process. 2015; 96: 211-220. https://doi.org/10.1016/j.fbp.2015.08.005
Jamie A, Alshami AS, Maliabari ZO, Ateih MA, Al Hamouz OCS. Immobilization and Enhanced Catalytic Activity of Lipase on Modified MWCNT for Oily Wastewater Treatment. Environ Prog Sustain Energy. 2016; 35(5): 1441-1449. https://doi.org/10.1002/ep
Che Marzuki NH, Mahat NA, Huyop F, Buang NA, Wahab RA. Candida rugosa Lipase Immobilized onto Acid-Functionalized Multi-walled Carbon Nanotubes for Sustainable Production of Methyl Oleate. Appl Biochem Biotechnol. 2015; 177(4): 967-984. https://doi.org/10.1007/s12010-015-1791-z
Mohamad NR, Buang NA, Mahat NA, Jamalis J, Huyop F, Aboul-Enein HY, Wahab RA. Simple adsorption of Candida rugosa lipase onto multi-walled carbon nanotubes for sustainable production of the flavor ester geranyl propionate. J Ind Eng Chem. 2015; 32: 99-108. https://doi.org/10.1016/j.jiec.2015.08.001
Mokhtarifar M, Arab H, Maghrebi M, Baniadam M. Amine-functionalization of carbon nanotubes assisted by electrochemical generation of chlorine. Appl Phys A Mater Sci Process. 2018; 124(1): 1-9. https://doi.org/10.1007/s00339-017-1438-8
Yook JY, Jun J, Kwak S. Amino functionalization of carbon nanotube surfaces with NH 3 plasma treatment. Appl Surf Sci. 2010; 256(23): 6941-6944. https://doi.org/10.1016/j.apsusc.2010.04.075
Asmat S, Anwer AH, Husain Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int J Biol Macromol. 2019; 140: 484-495. https://doi.org/10.1016/j.ijbiomac.2019.08.086
Raghavendra T, Basak A, Manocha LM, Shah AR, Madamwar D. Robust nanobioconjugates of Candida antarctica lipase B - Multiwalled carbon nanotubes: Characterization and application for multiple usages in non-aqueous biocatalysis. Bioresour Technol. 2013; 140: 103-110. https://doi.org/10.1016/j.biortech.2013.04.071
Rastian Z, Khodadadi AA, Guo Z, Vahabzadeh F, Mortazavi Y. Plasma Functionalized Multiwalled Carbon Nanotubes for Immobilization of Candida antarctica Lipase B: Production of Biodiesel from Methanolysis of Rapeseed Oil. Appl Biochem Biotechnol.2016; 178(5): 974-989. https://doi.org/10.1007/s12010-015-1922-6
Salah LS, Ouslimani N, Bousba D, Huynen I, Danleé Y, Aksas H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J Nanomater. 2021; 2021: 4972770. https://doi.org/10.1155/2021/4972770
Thakur CK, Karthikeyan C, Abou-Dahech MS, Altabakha MMAM, Al Shahwan MJS, Ashby CR, Tiwari AK, Babu RJ, Moorthy NSHN. Microwave-Assisted Functionalization of Multi-Walled Carbon Nanotubes for Biosensor and Drug Delivery Applications. Pharmaceutics. 2023; 15(2): 335. https://doi.org/10.3390/pharmaceutics15020335
Bié J, Sepodes B, Fernandes PCB, Ribeiro MHL. Enzyme Immobilization and Co-Immobilization: Main Framework, Advances and Some Applications. Processes. 2022; 10(3): 1-31. https://doi.org/10.3390/pr10030494
Smith S, Goodge K, Delaney M, Struzyk A, Tansey N, Frey M. A comprehensive review of the covalent immobilization of biomolecules onto electrospun nanofibers. Nanomaterials. 2020; 10(11): 1-39. https://doi.org/10.3390/nano10112142
Bourkaib MC, Guiavarc’h Y, Chevalot I, Delaunay S, Gleize J, Ghanbaja J, Valsaque F, Berrada N, Desforges A, Vigolo B. Non-covalent and covalent immobilization of Candida antarctica lipase B on chemically modified multiwalled carbon nanotubes for a green acylation process in supercritical CO2. Catal Today. 2020; 348: 26-36. https://doi.org/10.1016/j.cattod.2019.08.046
Dwivedee BP, Bhaumik J, Rai SK, Laha JK, Banerjee UC. Development of nanobiocatalysts through the immobilization of Pseudomonas fluorescens lipase for applications in efficient kinetic resolution of racemic compounds. Bioresour Technol. 2017; 239: 464-471. https://doi.org/10.1016/j.biortech.2017.05.050
Boncel S, Zniszczoł A, Szymańska K, Mrowiec-Białoń J, Jarzebski A, Walczak KZ. Alkaline lipase from Pseudomonas fluorescens non-covalently immobilised on pristine versus oxidised multi-wall carbon nanotubes as efficient and recyclable catalytic systems in the synthesis of Solketal esters. Enzyme Microb Technol. 2013; 53(4): 263-270. https://doi.org/10.1016/j.enzmictec.2013.05.003
Deep A, Sharma AL, Kumar P. Lipase immobilized carbon nanotubes for conversion of Jatropha oil tofatty acid methyl esters. Biomass and Bioenergy. 2015; 81: 83-87. https://doi.org/10.1016/j.biombioe.2015.06.008
Tan H, Feng W, Ji P. Lipase immobilized on magnetic multi-walled carbon nanotubes. Bioresour Technol. 2012; 115: 172-176. https://doi.org/10.1016/j.biortech.2011.10.066
Prlainović NZ, Bezbradica DI, Rogan JR, Uskoković PS, Mijin D, Marinković AD. Surface functionalization of oxidized multi-walled carbon nanotubes: Candida rugosa lipase immobilization. Comptes Rendus Chim. 2016; 19(3): 363-370. https://doi.org/10.1016/j.crci.2015.10.008
Ji P, Tan H, Xu X, Feng W. Lipase Covalently Attached to Multiwalled Carbon Nanotubes as an Efficient Catalyst in Organic Solvent. AIChE J. 2010; 56(11): 3005-3011. https://doi.org/10.1002/aic
Szelwicka A, Wolny A, Grymel M, Jurczyk S, Boncel S, Chrobok A. Chemo-enzymatic baeyer-villiger oxidation facilitated with lipases immobilized in the supported ionic liquid phase. Materials (Basel). 2021; 14(13): 3443. https://doi.org/10.3390/ma14133443
Szelwicka A, Erfurt K, Jurczyk S, Boncel S, Chrobok A. Outperformance in acrylation: Supported d-glucose-based ionic liquid phase on mwcnts for immobilized lipase B from Candida antarctica as catalytic system. Materials (Basel). 2021; 14(11): 3090. https://doi.org/10.3390/ma14113090
Wang L, Liu X, Jiang Y, Zhou L, Ma L, He Y, Gao J. Biocatalytic pickering emulsions stabilized by lipase-immobilized carbon nanotubes for biodiesel production. Catalysts. 2018; 8(12): 587. https://doi.org/10.3390/catal8120587
Verma ML, Naebe M, Barrow CJ, Puri M. Enzyme Immobilisation on Amino-Functionalised Multi-Walled Carbon Nanotubes: Structural and Biocatalytic Characterisation. PLoS One. 2013; 8(9): 16-18. https://doi.org/10.1371/journal.pone.0073642
Pavlidis I V., Vorhaben T, Tsoufis T, Rudolf P, Bornscheuer UT, Gournis D, Stamatis H. Development of effective nanobiocatalytic systems through the immobilization of hydrolases on functionalized carbon-based nanomaterials. Bioresour Technol. 2012; 115: 164-171. https://doi.org/10.1016/j.biortech.2011.11.007
Fan Y, Wu G, Su F, Li K, Xu L, Han X, Yan Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel. 2016; 178: 172-178. https://doi.org/10.1016/j.fuel.2016.03.071
Fan Y, Su F, Li K, Ke C, Yan Y. Carbon nanotube filled with magnetic iron oxide and modified with polyamidoamine dendrimers for immobilizing lipase toward application in biodiesel production. Sci Rep. 2017; 7: 1-13. https://doi.org/10.1038/srep45643
Malaibari ZO. Effect of MWCNTs surface properties on lipase immobilization and its catalytic activity. Mater Express. 2018; 8(2): 123-132. https://doi.org/10.1166/mex.2018.1414
Badgujar KC, Sasaki T, Bhanage BM. Synthesis of lipase nano-bio-conjugates as an efficient biocatalyst: Characterization and activity-stability studies with potential biocatalytic applications. RSC Adv. 2015; 5: 55238-55251. https://doi.org/10.1039/C5RA10032A
Bartha-Vári JH, Moisă ME, Bencze LC, Irimie FD, Paizs C, Toșa MI. Efficient biodiesel production catalyzed by nanobioconjugate of lipase from Pseudomonas fluorescens. Molecules. 2020; 25(3): 651. https://doi.org/10.3390/molecules25030651
Kapoor M, Gupta MN. Lipase promiscuity and its biochemical applications. Process Biochem. 2012; 47(4): 555-569. https://doi.org/10.1016/j.procbio.2012.01.011
Al-Zuhair S, Ling FW, Jun LS. Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochem. 2007; 42(6): 951-960. https://doi.org/10.1016/j.procbio.2007.03.002
Brzozowski AM, Derewenda U, Derewenda ZS, Dodson GG, Lawson DM, Turkenburg JP, Bjorkling F, Huge-Jensen B, Patkar SA, Thim L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature. 1991; 351(6326): 491-494. https://doi.org/10.1038/351491a0
Adlercreutz P. Immobilisation and application of lipases in organic media. Chem Soc Rev. 2013; 42(15): 6406-6436. https://doi.org/10.1039/c3cs35446f
Sankaran R, Show PL, Chang J-S. Biodiesel production using immobilized lipase: feasibility and challenges. Biofuels, Bioprod Biorefining. 2016; 10(6): 896-916. https://doi.org/10.1002/BBB
Zhong L, Feng Y, Wang G, Wang Z, Bilal M, Lv H, Jia S, Cui J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int J Biol Macromol. 2020; 152: 207-222. https://doi.org/10.1016/j.ijbiomac.2020.02.258
Idris A, Bukhari A. Immobilized Candida antarctica lipase B: Hydration, stripping off and application in ring opening polyester synthesis. Biotechnol Adv. 2012; 30(3): 550-563. https://doi.org/10.1016/j.biotechadv.2011.10.002
Singh N, Dhanya BS, Verma ML. Nano-immobilized biocatalysts and their potential biotechnological applications in bioenergy production. Mater Sci Energy Technol. 2020; 3: 808-824. https://doi.org/10.1016/j.mset.2020.09.006
Basso A, Serban S. Industrial applications of immobilized enzymes—A review. Mol Catal. 2019; 479: 110607. https://doi.org/10.1016/j.mcat.2019.110607
Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev. 2012; 16(4): 2070-2093. https://doi.org/10.1016/j.rser.2012.01.003
Hama S, Noda H, Kondo A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr Opin Biotechnol. 2018; 50: 57-64. https://doi.org/10.1016/j.copbio.2017.11.001
Quayson E, Amoah J, Hama S, Kondo A, Ogino C. Immobilized lipases for biodiesel production: Current and future greening opportunities. Renew Sustain Energy Rev. 2020; 134: 110355. https://doi.org/10.1016/j.rser.2020.110355
Avhad MR, Marchetti JM. Uses of enzymes for biodiesel production. In: Hoseini M, ed. Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals and Bioproducts. Woodhead Publishing, Elsevier; 2019; 135-152. https://doi.org/10.1016/B978-0-12-817941-3.00007-3
Tacias-Pascacio VG, Torrestiana-Sánchez B, Dal Magro L, Virgen-Ortíz JJ, Suárez-Ruíz FJ, Rodrigues RC, Fernandez-Lafuente R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew Energy. 2019; 135: 1-9. https://doi.org/10.1016/J.RENENE.2018.11.107
Shim M, Kam NWS, Chen RJ, Li Y, Dai H. Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition. Nano Lett. 2002; 2(4): 285-288. https://doi.org/10.1021/nl015692j
Bayout I, Bouzemi N, Guo N, Mao X, Serra S, Riva S, Secundo F. Natural flavor ester synthesis catalyzed by lipases. Flavour Fragr J. 2020; 35(2): 209-218. https://doi.org/10.1002/ffj.3554
Liu YQ, WeiZhuo X, Wei X. A review on lipase-catalyzed synthesis of geranyl esters as flavor additives for food, pharmaceutical and cosmetic applications. Food Chem Adv. 2022; 1: 100052. https://doi.org/10.1016/j.focha.2022.100052
Muralidhar R, Marchant R, Nigam P. Lipases in racemic resolutions. J Chem Technol Biotechnol. 2001; 76(1): 3-8. https://doi.org/10.1002/1097-4660(200101)76:1<3::AID-JCTB336>3.0.CO;2-8
Cammarota MC, Teixeira GA, Freire DMG. Enzymatic pre-hydrolysis and anaerobic degradation of wastewaters with high fat contents. Biotechnol Lett. 2001; 23(19): 1591-1595. https://doi.org/10.1023/A:1011973428489
Scioli C, Vollaro L. The use of Yarrowia lipolytica to reduce pollution in olive mill wastewaters. Water Res. 1997; 31(10): 2520-2524. https://doi.org/10.1016/S0043-1354(97)00083-3
Masse L, Massé DI, Kennedy KJ. Effect of hydrolysis pretreatment on fat degradation during anaerobic digestion of slaughterhouse wastewater. Process Biochem. 2003; 38(9): 1365-1372. https://doi.org/10.1016/S0032-9592(03)00020-7
Masse L, Kennedy KJ, Chou S. Testing of alkaline and enzymatic hydrolysis pretreatments for fat particles in slaughterhouse wastewater. Bioresour Technol. 2001; 77(2): 145-155. https://doi.org/10.1016/S0960-8524(00)00146-2
MongkolthanarukW, DharmsthitiS. Biodegradation of lipid-rich wastewater by a mixed bacterial consortium Biodegradation of lipid-rich wastewater by a mixed bacterial consortium. Int Biodeterior Biodegrad. 2012; 50: 101-105. https://doi.org/10.1016/S0964-8305(02)00057-4
Jeganathan J, Bassi A, Nakhla G. Pre-treatment of high oil and grease pet food industrial wastewaters using immobilized lipase hydrolyzation. J Hazard Mater. 2006; 137(1): 121-128. https://doi.org/10.1016/j.jhazmat.2005.11.106
Liu J, Zhao W, Zhang L, Zhang M, Chen Y, Xu Y, Li Y, Wang L. Synthesis of substituted 2H-chromenes catalyzed by lipase immobilized on magnetic multiwalled carbon nanotubes. Biotechnol Appl Biochem. 2021; 68(2): 411-416. https://doi.org/10.1002/bab.1939