Toxic dye removal by thermally modified lignocellulosic waste in a three-phase air-lift reactor: Kinetic insights Original scientific paper
Main Article Content
Abstract
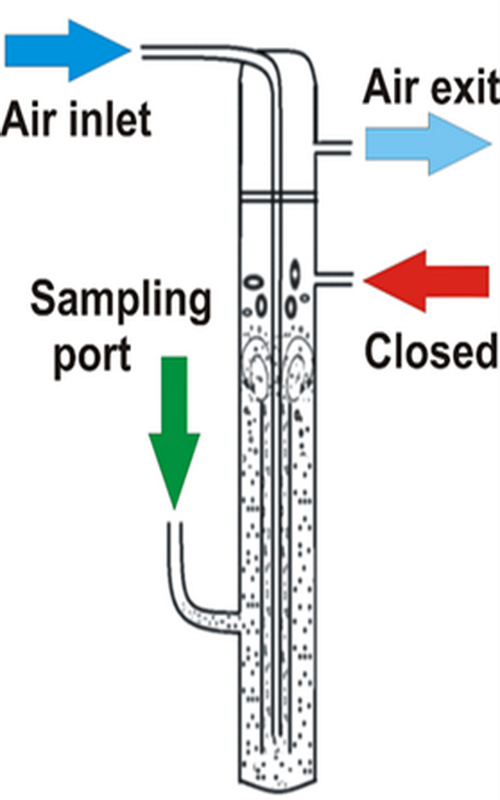
This paper investigates the influence of the air flow rate in a three-phase air-lift reactor on the sorption of toxic dye, Brilliant green, onto a promising and efficient sorbent, sour cherry stone biochar. In order to gain a comprehensive insight into the sorbent/sorption behaviour, sour cherry stone biochar was characterized by Fourier transform infrared spectroscopy with attenuated total reflection, pH of the suspension, point of zero charge, scanning electron microscopy with energy-dispersive X-ray spectroscopy and X-ray diffraction. The experiments were performed in an air-lift reactor using airflows of 2.50 and 5.55 dm3 h-1. The experimental data of sorption kinetics experiments were fitted by non-linear form of pseudo-first and, pseudo-second models as well as the Weber-Morris model based on intraparticle diffusion. The overall sorption rate was found to be limited by the Brilliant Green mass transport rate to the sorbent at a lower airflow and thus mixing intensity, while it was kinetically controlled at a higher rate following the pseudo-second order kinetic model. Furthermore, sorption at lower air flow was delayed by mass transfer resistance through the liquid boundary layer surrounding sorbent particles. Presented results clearly indicate that airflow intensity plays a significant role in the overall sorption kinetics and support possible application of the applied biochar for efficient Brilliant Green sorption.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200023 -
Science Fund of the Republic of Serbia
Grant numbers 7439
References
Elgarahy AM, Elwakeel KZ, Mohammad SH, Elshoubaky GA. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean Eng Technol. 2021; 4: 100209. https://doi.org/10.1016/j.clet.2021.100209
Anastopoulos I, Ahmed MJ, Hummadi EH. Eucalyptus-based materials as adsorbents for heavy metals and dyes removal from (waste)waters. J Mol Liq. 2022; 356: 118864. https://doi.org/10.1016/j.molliq.2022.118864
Siregar Global dyes & pigments market size report, 2021-2028. https://www.grandviewresearch.com/industry-analysis/dyes-and-pigments-market. Accessed January 7, 2023.
Fiaz R, Hafeez M, Mahmood R. Removal of brilliant green (BG) from aqueous solution by using low cost biomass salix alba leaves (SAL): Thermodynamic and kinetic studies. J Water Reuse Desalin. 2020; 10(1) :70-81. https://doi.org/10.2166/wrd.2020.054
Vyavahare G, Gurav R, Patil R, Sutar S, Jadhav P, Patil D, Yang YH, Tang J, Chavan C, Kale S, Jadhav J. Sorption of brilliant green dye using soybean straw-derived biochar: characterization, kinetics, thermodynamics and toxicity studies. Environ Geochem Health. 2021; 43(8): 2913-26. https://doi.org/10.1007/s10653-020-00804-y
Bayramoglu G, Altintas B, Arica MY. Adsorption kinetics and thermodynamic parameters of cationic dyes from aqueous solutions by using a new strong cation-exchange resin. Chem Eng J. 2009; 152(2-3): 339-346. https://doi.org/10.1016/j.cej.2009.04.051
Antanasković A, Lopičić Z, Pehlivan E, Adamović V, Šoštarić T, Milojković J, Milivojević M. Thermochemical conversion of non-edible fruit waste for dye removal from wastewater. Biomass Convers Biorefinery. 2023; (0123456789). https://doi.org/10.1007/s13399-023-04083-2
Haskis P, Tsolis P, Tsiantouka L, Mpeza P, Barouchas P, Giannopoulos G, Pashalidis Ι, Anastopoulos Ι. Biosorption of Methylene Blue dye by Ligustrum lucidum fruits biomass: Equilibrium, isotherm, kinetic and thermodynamic studies Panagiotis. Glob NEST J. 2023; 25: 97-104. https://doi.org/https://doi.org/10.30955/gnj.005294
Foo KY, Hameed BH. An overview of dye removal via activated carbon adsorption process. Desalin Water Treat. 2010; 19(1-3): 255-274. https://doi.org/10.5004/dwt.2010.1214
Barquilha CER, Braga MCB. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour Technol Reports. 2021; 15: 100728. https://doi.org/10.1016/j.biteb.2021.100728
Ahmed MJ, Danish M, Anastopoulos I, Iwuozor KO. Recent progress on corn (Zea mays L.)-based materials as raw, chemically modified, carbonaceous, and composite adsorbents for aquatic pollutants: A review. J Anal Appl Pyrolysis 2023; 172(2): 106004. https://doi.org/10.1016/j.jaap.2023.106004
Srivatsav P, Bhargav BS, Shanmugasundaram V, Arun J, Gopinath KP, Bhatnagar A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: A review. Water. 2020; 12(12): 3561. https://doi.org/10.3390/w12123561
Lopičić Z, Avdalović J, Milojković J, Antanasković A, Lješević M, Lugonja N, Šoštarić T. Removal of diesel pollution by biochar - support in water remediation. Hem Ind. 2021; 75(6): 329-39. https://doi.org/10.2298/HEMIND210514029L
Statistical office of the Republic of Serbia. https://www.stat.gov.rs/en-us/. Accessed May 12, 2023.
Milivojević M, Andrejić D, Bugarski B. Effects of air-lift reactor dimensions on its hydrodinamic characteristics. Hem Ind. 2010; 64(1): 35-46. https://doi.org/10.2298/HEMIND1009035M
Milivojevic M, Pavlou S, Pajic-Lijakovic I, Bugarski B. Dependence of slip velocity on operating parameters of air-lift bioreactors. Chem Eng J. 2007; 132(1-3): 117-23. https://doi.org/10.1016/j.cej.2007.01.026
Jones SMJ, Harrison STL. Aeration energy requirements for lipid production by Scenedesmus sp. in airlift bioreactors. Algal Res 2014; 5(1): 249-57. https://doi.org/10.1016/j.algal.2014.03.003
Cerri MO, Badino AC. Shear conditions in clavulanic acid production by Streptomyces clavuligerus in stirred tank and airlift bioreactors. Bioprocess Biosyst Eng. 2012; 35(6): 977-84. https://doi.org/10.1007/s00449-012-0682-8
Milivojevic M, Pavlou S, Bugarski B. Liquid velocity in a high-solids-loading three-phase external-loop airlift reactor. J Chem Technol Biotechnol. 2012; 87(11): 1529-40. https://doi.org/10.1002/jctb.3783
Veljković M, Simović M, Banjanac K, Ćorović M, Milivojević A, Milivojević M, Bezbradica D. Heterofunctional epoxy support development for immobilization of fructosyltransferase from Pectinex® Ultra SP-L: batch and continuous production of fructo-oligosaccharides. React Chem Eng. 2022; 7(12): 2518-26. https://doi.org/10.1039/d2re00182a
Veljković M, Stepanović R, Banjanac K, Ćorović M, Milivojević A, Simović M, Milivojević M, Bezbradica D. Continuous production of fructo-oligosaccharides using selectively immobilized fructosyltransferase from Aspergillus aculeatus onto Purolite® A109. J Ind Eng Chem. 2023; 117: 149-56. https://doi.org/10.1016/j.jiec.2022.09.051
Milonjić SK, Ruvarac AL, Šušić M V. The heat of immersion of natural magnetite in aqueous solutions. Thermochim Acta. 1975; 11(3): 261-266. https://doi.org/10.1016/0040-6031(75)85095-7
Lagergren S. About the theory of so called adsorption of soluble substances. K Sven Veternskapsakad Handl. 1898; 24:1-39.
Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34: 451-465.
Weber, W.J. Morris JC. Kinetics of adsorption on carbon from solution. J Sanit Eng Div. 1963; 89: 31-60.
Behazin E, Ogunsona E, Rodriguez-Uribe A, Mohanty AK, Misra M, Anyia AO. Mechanical, chemical, and physical properties of wood and perennial grass biochars for possible composite application. BioResources. 2016 ;11(1): 1334-1348. https://doi.org/10.15376/biores.11.1.1334-1348
Sakhiya AK, Vijay VK, Kaushal P. Efficacy of rice straw derived biochar for removal of Pb+2 and Zn+2 from aqueous: Adsorption, thermodynamic and cost analysis. Bioresour Technol Reports. 2022; 17(6): 100920. https://doi.org/10.1016/j.biteb.2021.100920
Ukkund SJ, Puthiyillam P, Alshehri HM, Goodarzi M, Taqui SN, Anqi AE, Safaei MR, Ali MA, Syed UT, Mir RA, Elfasakhany A, Eed EM, Siddiqui MIH, Mokashi I, Soudagar MEM. Adsorption method for the remediation of brilliant green dye using halloysite nanotube: Isotherm, kinetic and modeling studies. Appl Sci. 2021; 11(17): 8088. https://doi.org/10.3390/app11178088
Keiluweit M, Nico PS, Johnson M, Kleber M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol. 2010; 44(4): 1247-1253. https://doi.org/10.1021/es9031419
Paunovic O, Pap S, Maletic S, Taggart MA, Boskovic N, Turk Sekulic M. Ionisable emerging pharmaceutical adsorption onto microwave functionalised biochar derived from novel lignocellulosic waste biomass. J Colloid Interface Sci. 2019; 547: 350-360. https://doi.org/10.1016/j.jcis.2019.04.011
Saghir S, Pu C, Fu E, Wang Y, Xiao Z. Synthesis of high surface area porous biochar obtained from pistachio shells for the efficient adsorption of organic dyes from polluted water. Surfaces and Interfaces. 2022; 34 :102357. https://doi.org/10.1016/j.surfin.2022.102357
Guilhen SN, Watanabe T, Silva TT, Rovani S, Marumo JT, Tenório JAS, Mašek O, Araujo LG de. Role of Point of Zero Charge in the Adsorption of Cationic Textile Dye on Standard Biochars from Aqueous Solutions: Selection Criteria and Performance Assessment. Recent Prog Mater. 2022; 4(2). https://doi.org/10.21926/rpm.2202010
Kahraman HT, Pehlivan E. Cr6 + removal using oleaster (Elaeagnus) seed and cherry (Prunus avium) stone biochar. Powder Technol. 2017; 306: 61-67. https://doi.org/10.1016/j.powtec.2016.10.050
Chen T, Liu R, Scott NR. Characterization of energy carriers obtained from the pyrolysis of white ash, switchgrass and corn stover - Biochar, syngas and bio-oil. Fuel Process Technol. 2016; 142: 124-134. https://doi.org/10.1016/j.fuproc.2015.09.034
Zhang S, Wang J. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue-derived biochar. Water Air Soil Pollut. 2021; 232(6): 236. https://doi.org/10.1007/s11270-021-05193-1
Einfal T, Planinšek O, Hrovat K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013; 63(3): 305-334. https://doi.org/10.2478/acph-2013-0026
Aljeboree AM, Alshirifi AN, Alkaim AF. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem. 2017; 10: S3381-S3393. https://doi.org/10.1016/j.arabjc.2014.01.020
Nassar M, Farrag T. Kinetics and Process Design for Adsorption of Maxilon Red Dye From Aqueous Solutions Using Gas Mixing. Int Conf Chem Environ Eng. 2012; 6(6): 1-13. https://doi.org/10.21608/iccee.2012.35794
Giri BS, Gun S, Pandey S, Trivedi A, Kapoor RT, Singh RP, Abdeldayem OM, Rene ER, Yadav S, Chaturvedi P, Sharma N, Singh RS. Reusability of brilliant green dye contaminated wastewater using corncob biochar and Brevibacillus parabrevis: hybrid treatment and kinetic studies. Bioengineered. 2020; 11(1): 743-758. https://doi.org/10.1080/21655979.2020.1788353
Sukla Baidya K, Kumar U. Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk. South African J Chem Eng. 2021; 35: 33-43. https://doi.org/10.1016/j.sajce.2020.11.001
Fadali OA. Effect of gas strirring on external mass transfer, intraparticle diffusion and energy consumption during adsorption. Adsorpt Sci Technol. 2003; 21(10): 935-950. https://doi.org/10.1260/02636170360744371
Obradovic B. Guidelines for general adsorption kinetics modeling. Hem Ind. 2020; 74(1): 65-70. https://doi.org/10.2298/HEMIND200201006O
McKay G. the Adsorption of Dyestuffs From Aqueous Solutions Using Activated Carbon. Iii. Intraparticle Diffusion Processes. J Chem Technol Biotechnol Chem Technol. 1983; 33 A(4): 196-204. https://doi.org/10.1002/jctb.504330406
Zhu Q, Moggridge GD, D’Agostino C. Adsorption of pyridine from aqueous solutions by polymeric adsorbents MN 200 and MN 500. Part 2: Kinetics and diffusion analysis. Chemical Engineering Journal. 2016; 306: 1223-1233. https://doi.org/10.1016/j.cej.2016.07.087
McKay G, Otterburn MS, Sweeney AG. The removal of colour from effluent using various adsorbents-III. Silica: Rate processes. Water Res. 1980; 14(1): 15-20. https://doi.org/10.1016/0043-1354(80)90037-8
Selambakkannu S, Othman NAF, Bakar KA, Karim ZA. Adsorption studies of packed bed column for the removal of dyes using amine functionalized radiation induced grafted fiber. SN Appl Sci. 2019; 1(2): 175. https://doi.org/10.1007/s42452-019-0184-2
Pérez-Cadena R, García-Esquivel Y, Castañeda-Cisneros YE, Serna-Díaz MG, Ramírez-Vargas MR, Muro-Urista CR, Téllez-Jurado A. Biological decolorization of Amaranth dye with Trametes polyzona in an airlift reactor under three airflow regimes. Heliyon. 2020; 6(12). https://doi.org/10.1016/j.heliyon.2020.e05857
Saif Ur Rehman M, Kim I, Rashid N, Adeel Umer M, Sajid M, Han JI. Adsorption of Brilliant Green Dye on Biochar Prepared From Lignocellulosic Bioethanol Plant Waste. Clean - Soil, Air, Water. 2016; 44(1): 55-62. https://doi.org/10.1002/clen.201300954