Photodegradation of thiophanate-methyl under simulated sunlight by utilization of novel composite photocatalysts Original scientific paper
Main Article Content
Abstract
This work aimed to investigate the influence of modified titanium(IV) oxide by different nanosized particles on photocatalytic capacity to decompose the chosen organic pollutant under simulated sunlight. For that purpose, rutile-phased titanium(IV) oxide (r-TiO2) was decorated with iron vanadate (FeVO4/r-TiO2) and vanadium-substituted goethite
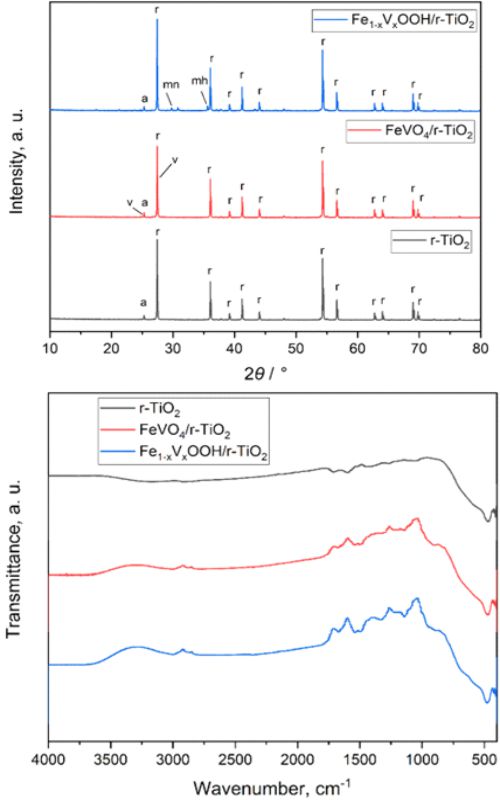
(Fe1-xVxOOH/r-TiO2). The obtained composites were characterized by field emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, X ray powder diffraction, Brunauer-Emmett-Teller, Fourier transform infrared spectroscopy – attenuated total reflectance and ultraviolet–visible diffuse reflectance spectroscopy techniques. Both synthesized photocatalysts showed higher photoactivity than the base r-TiO2 for the degradation of the target contaminant - thiophanate-methyl (2.5 h vs. 5 h). During the tests, parameters like the irradiation time, catalysts amount, and pesticide concentration were systematically investigated. Furthermore, photocatalysts were applied in multicycle degradation tests for examining their effectiveness during exploitation time. Monitoring of the removal rate was performed both by UV/visible spectrometry and high-performance liquid chromatography (HPLC). In order to prove completion of fungicide degradation chemical oxygen demand was measured in the course of the photocatalytic experiment. The final concentration of the observed contaminant in treated samples was under the prescribed legislative level. The fabricated materials displayed great reliability, durability and photocatalytic activity representing good potentials for implementing this process in real wastewater treatment plants.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200023; 451-03-9/2023-14/200017; 451-03-47/2023-01/200135;451-03-47/2023-01/200023;451-03-9/2023-14/200017;451-03-47/2023-01/200135
References
Schaider LA, Rodgers KM, Rudel RA. Review of Organic Wastewater Compound Concentrations and Removal in Onsite Wastewater Treatment Systems. Environ Sci Technol. 2017; 51(13): 7304-7317. https://doi.org/10.1021/acs.est.6b04778
Syafrudin M, Kristanti RA, Yuniarto A, Hadibarata T, Rhee J, Al-Onazi WA, Algarni TS, Almarri AH, Al-Mohaimeed AM. Pesticides in drinking water-a review. Int J Environ Res Public Health. 2021; 18(2): 468. https://doi.org/10.3390/ijerph18020468
Hassaan MA, El Nemr A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt J Aquat Res. 2020; 46(3): 207-220. https://doi.org/10.1016/j.ejar.2020.08.007
Jatoi AS, Hashmi Z, Adriyani R, Yuniarto A, Mazari SA, Akhter F, Mubarak NM. Recent trends and future challenges of pesticide removal techniques – A comprehensive review. J Environ Chem Eng. 2021; 9(4): 105571. https://doi.org/10.1016/j.jece.2021.105571
Leong WH, Teh SY, Hossain MM, Nadarajaw T, Zabidi-Hussin Z, Chin SY, Lai KS, Lim SHE. Application, monitoring and adverse effects in pesticide use: The importance of reinforcement of Good Agricultural Practices (GAPs). J Environ Manage. 2020; 260 109987. https://doi.org/10.1016/j.jenvman.2019.109987
Venugopal D, Karunamoorthy P, Beerappa R, Sharma D, Aambikapathy M, Rajasekar K, Gaikwad A, Kondhalkar S. Evaluation of work place pesticide concentration and health complaints among women workers in tea plantation, Southern India. J Expo Sci Environ Epidemiol. 2021; 31(3): 560-570. https://doi.org/10.1038/s41370-020-00284-3
Che X, Huang Y, Zhong K, Jia K, Wei Y, Meng Y, Yuan W, Lu H. Thiophanate-methyl induces notochord toxicity by activating the PI3K-mTOR pathway in zebrafish (Danio rerio) embryos. Environ Pollut. 2023; 318: 120861. https://doi.org/10.1016/j.envpol.2022.120861
Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A, Villamar-Bouza L. Peer review of the pesticide risk assessment of the active substance thiophanate-methyl. EFSA J. 2018; 16(5): e05133. https://doi.org/10.2903/j.efsa.2018.5133
European Commission, Commission Implementing Regulation (EU) 2020/1498 concerning the non-renewal of approval of the active substance thiophanate-methyl, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJEU. 2020; https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R1498 (accessed December 12, 2022)
Tan H, Li Q, Zhang H, Wu C, Zhao S, Deng X, Li Y. Pesticide residues in agricultural topsoil from the Hainan tropical riverside basin: Determination, distribution, and relationships with planting patterns and surface water. Sci Tot Environ. 2020; 722: 137856. https://doi.org/10.1016/j.scitotenv.2020.137856
Saleh IA, Zouari N, Al-Ghouti MA. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ Technol Innov. 2020; 19: . https://doi.org/10.1016/j.eti.2020.101026
Mukherjee A, Mehta R, Saha S, Bhattacharya A, Biswas PK, Kole RK. Removal of multiple pesticide residues from water by low-pressure thin-film composite membrane. Appl Water Sci. 2020; 10(12): 1-8. https://doi.org/10.1007/s13201-020-01315-y
Jovanović AA, Bugarčić MD, Marinković AD, Sokić MD. Insights into the application of polyaniline-based composites in environmental engineering. Metal Mater Data. 2023; 1(1): 25-31. https://doi.org/10.30544/MMD1
Zhang F, Wang X, Liu H, Liu C, Wan Y, Long Y, Cai Z. Recent advances and applications of semiconductor photocatalytic technology. Appl Sci. 2019; 9(12): 1-43. https://doi.org/10.3390/app9122489
Shokri A, Sanavi Fard M. A critical review in the features and application of photocatalysts in wastewater treatment. Chem Paper. 2022; 76(9): 5309-5339. https://doi.org/10.1007/s11696-022-02256-3
Zhang Y, Chu W. Cooperation of multi-walled carbon nanotubes and cobalt doped TiO2 to activate peroxymonosulfate for antipyrine photocatalytic degradation. Sep Purif Technol. 2022; 282: 119996. https://doi.org/10.1016/j.seppur.2021.119996
He X, Kai T, Ding P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: a review. Environ Chem Lett. 2021; 19(6): 4563-4601. https://doi.org/10.1007/s10311-021-01295-8
Low J, Yu J, Jaroniec M, Wageh S, Al-Ghamdi AA. Heterojunction Photocatalysts. Adv Mat. 2017; 29(20): 1601694. https://doi.org/10.1002/adma.201601694
Xu J, Zhang T. Fabrication of spent FCC catalyst composites by loaded V2O5 and TiO2 and their comparative photocatalytic activities. Sci Rep. 2019; 9(1):11099. https://doi.org/10.1038/s41598-019-47155-y
Kesavan G, Pichumani M, Chen SM. Influence of Crystalline, Structural, and Electrochemical Properties of Iron Vanadate Nanostructures on Flutamide Detection. ACS Appl Nano Mater. 2021; 4(6): 5883-5894. https://doi.org/10.1021/acsanm.1c00802
Dutta DP, Ramakrishnan M, Roy M, Kumar A. Effect of transition metal doping on the photocatalytic properties of FeVO4 nanoparticles. J Photochem Photobiol A Chem. 2017; 335: 102-111. https://doi.org/10.1016/j.jphotochem.2016.11.022
Min YL, Zhang K, Chen YC, Zhang YG. Synthesis of novel visible light responding vanadate/TiO2 heterostructure photocatalysts for application of organic pollutants. Chem Eng J. 2011; 175(1): 76-83. https://doi.org/10.1016/j.cej.2011.09.042
Schwertman U, Cornell RM. Iron Oxides in the Laboratory. Second edition. Weinheim: Wiley VCH Verlag GmbH; 2000, 91-92.
Ines M, Paolo P, Roberto F, Mohamed S. Experimental studies on the effect of using phase change material in a salinity-gradient solar pond under a solar simulator. Sol Energy. 2019; 186: 335-346. https://doi.org/10.1016/j.solener.2019.05.011
EPA. Method 410.4, Revision 2.0: The Determination of Chemical Oxygen Demand by Semi-Automated Colorimetry. 1993
Ozer D, Tunca ET, Oztas NA. Effects of fuel type on iron vanadate nanocatalyst synthesized by solution combustion method for methylene blue degradation. J Nanopartcl Res. 2021; 23(8): 1- 12. https://doi.org/10.1007/s11051-021-05303-4
Zhao Y, Yao K, Cai Q, Shi Z, Sheng M, Lin H, Shao M. Hydrothermal route to metastable phase FeVO4 ultrathin nanosheets with exposed {010} facets: Synthesis, photocatalysis and gas-sensing. Cryst Eng Comm- 2014; 16(2): 270-276. https://doi.org/10.1039/c3ce41692e
Cui Y, Xue Y, Zhang R, Zhang J, Li X, Zhu X. Vanadium-cobalt oxyhydroxide shows ultralow overpotential for the oxygen evolution reaction. J Mater Chem A Mater. 2019; 7(38):21911-21917. https://doi.org/10.1039/c9ta07918a
Tamirat AG, Su WN, Dubale AA, Chen HM, Hwang BJ. Photoelectrochemical water splitting at low applied potential using a NiOOH coated codoped (Sn, Zr) α-Fe2O3 photoanode. J Mater Chem A Mater. 2015; 3(11): 5949-5961. https://doi.org/10.1039/c4ta06915c
Frison R, Cernuto G, Cervellino A, Zaharko O, Colonna GM, Guagliardi A, Masciocchi N. Magnetite-maghemite nanoparticles in the 5-15 nm range: Correlating the core-shell composition and the surface structure to the magnetic properties. A total scattering study. Chem Mat. 2013; 25(23): 4820-4827. https://doi.org/10.1021/cm403360f
Nithya VD, Selvan RK, Sanjeeviraja C, Radheep DM, Arumugam S. Synthesis and characterization of FeVO4 nanoparticles. Mater Res Bull. 2011; 46(10): 1654-1658. https://doi.org/10.1016/j.materresbull.2011.06.005
Zhu X, Chen J, Yu X, Zhu X, Gao X, Cen K. Controllable synthesis of novel hierarchical V2O5/TiO2 nanofibers with improved acetone oxidation performance. RSC Adv. 2015; 5(39): 30416-30424. https://doi.org/10.1039/c5ra01001b
Ghiyasiyan-Arani M, Salavati-Niasari M, Masjedi-Arani M, Mazloom F. An easy sonochemical route for synthesis, characterization and photocatalytic performance of nanosized FeVO4 in the presence of aminoacids as green capping agents. J Mater Sci: Mater Electron. 2018; 29(1): 474-485. https://doi.org/10.1007/s10854-017-7936-9
George S, Pokhrel S, Ji Z, Henderson BL, Xia T, Li L, Zink JI, Nel AE, Mädler L. Role of Fe doping in tuning the band gap of TiO2 for the photo-oxidation-induced cytotoxicity paradigm. J Am Chem Soc. 2011; 133(29): 11270-11278. https://doi.org/10.1021/ja202836s
Jovanović A, Stevanović M, Barudžija T, Cvijetić I, Lazarević S, Tomašević A, Marinković A. Advanced technology for photocatalytic degradation of thiophanate-methyl: Degradation pathways, DFT calculations and embryotoxic potential. Process Saf Environ Prot. 2023; 178: 423-443. https://doi.org/10.1016/j.psep.2023.08.054
Lewis KA, Tzilivakis J, Warner DJ, Green A. An international database for pesticide risk assessments and management. Hum Ecol Risk Assess. 2016; 22(4): 1050-1064. https://doi.org/10.1080/10807039.2015.1133242
Yoon H, Kim D, Park M, Kim J, Kim J, Srituravanich W, Shin B, Jung Y, Jeon S. Extraordinary Enhancement of UV Absorption in TiO2 Nanoparticles Enabled by Low-Oxidized Graphene Nanodots. J Phys Chem C. 2018; 122(22): 12114-12121. https://doi.org/10.1021/acs.jpcc.8b03329
Alamelu K, Jaffar Ali BM. TiO2-Pt composite photocatalyst for photodegradation and chemical reduction of recalcitrant organic pollutants. J Environ Chem Eng. 2018; 6(5): 5720-5731. https://doi.org/10.1016/j.jece.2018.08.042
Gonçalves JM, Ireno Da Silva M, Angnes L, Araki K. Vanadium-containing electro and photocatalysts for the oxygen evolution reaction: A review. J Mater Chem A Mater. 2020; 8(5): 2171-2206. https://doi.org/10.1039/c9ta10857b
Sindhu AS, Shinde NB, Harish S, Navaneethan M, Eswaran SK. Recoverable and reusable visible-light photocatalytic performance of CVD grown atomically thin MoS2 films. Chemosphere. 2022; 287: 132347. https://doi.org/10.1016/j.chemosphere.2021.132347
Guo T, Yang S, Chen Y, Yang L, Sun Y, Shang Q. Photocatalytic kinetics and cyclic stability of photocatalysts Fe-complex/TiO2 in the synergistic degradation of phenolic pollutants and reduction of Cr(VI). Environ Sci Pollut Res. 2021; 28(10): 12459-12473. https://doi.org/10.1007/s11356-020-11220-1
Sigma Aldrich 2023. https://www.sigmaaldrich.com/RS/en/search (accessed May 22, 2023)