Cobalt recovery from spent lithium-ion batteries by leaching in H2SO4-N2 and H2SO4-O2 systems followed by electrochemical deposition Original scientific paper
Main Article Content
Abstract
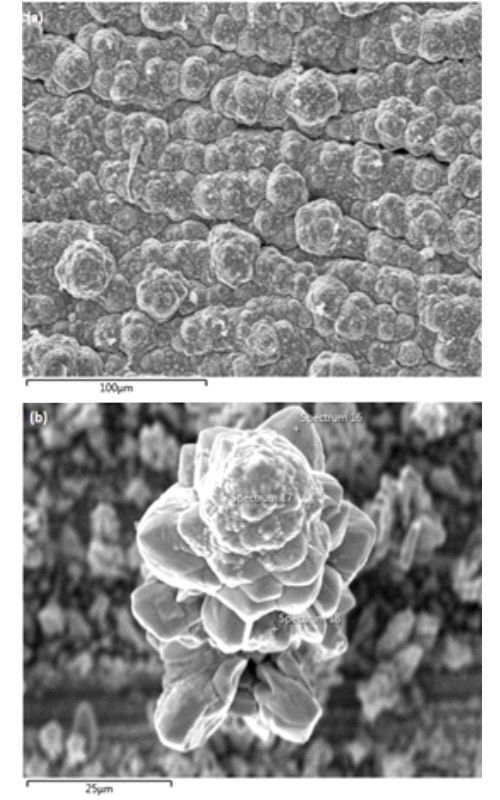
This paper is focused on cobalt valorization from the cathode material of spent lithium-ion batteries (LIBs) by using leaching and electrochemical deposition methods. During the leaching experiments, the degrees of cathode material dissolution in H2SO4-N2 and H2SO4-O2 systems were compared. Maximal degrees of cobalt extraction were 40 % in the former and 47 % in the latter system under following experimental conditions: H2SO4 concentration of 2 mol dm-3, nitrogen/oxygen volumetric flow of 2 L min-1, solid phase concentration of 33 g L-1, and temperature of 85 °C. The rate of cobalt extraction from the cathode material in both investigated systems was the most favorable in the first 15 min, after which there was a sudden decrease in the reaction rate. Cobalt from the leaching solution was deposited on a copper substrate by galvanostatic electrochemical deposition with a current efficiency of 84 %. The energy consumption was 5.8 kWh kg-1 of deposited Co. The cyclic voltammetry (CV) method was used to determine the potential of cobalt deposition, as well as side reactions taking place in the system. Scanning electron microscopy with energy dispersive spectrometry has shown that during the process of electrochemical deposition agglomeration of cobalt particles occurred (in the shape of cauliflower), while the metal was deposited in its elemental state, which was also confirmed by the results of X-ray diffraction analysis.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/ 20013;451-03-47/2023-01/ 200052
References
Guo Y, Zhao YL, Lou X, Zhou T, Wang Z, Fang C, Guan J, Chen S, Xu X, Zhang RQ. Efficient degradation of industrial pollutants with sulfur (IV) mediated by LiCoO2 cathode powders of spent lithium ion batteries: A “treating waste with waste” strategy. J Hazard Mater. 2020; 399: 123090. https://doi.org/10.1016/j.jhazmat.2020.123090
Chen X, Ma H, Luo C, Zhou T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J Hazard Mater. 2017; 326: 77−86. https://doi.org/10.1016/j.jhazmat.2016.12.021
Wang MM, Zhang CC, Zhang FS. Recycling of spent lithium-ion battery with polyvinyl chloride by mechano chemical process. Waste Manage. 2017; 67: 232−239. https://doi.org/10.1016/j.wasman.2017.05.013
Meng Q, Zhang Y, Dong P, Liang F. A novel process for leaching of metals from LiNi1/3Co1/3Mn1/3O2 material of spent lithium ion batteries: Process optimization and kinetics aspects. J Ind Eng Chem. 2018; 61: 133−141. https://doi.org/10.1016/j.jiec.2017.12.010
Yang Y, Sun W, Bu Y, Zhang C, Song S, Hu Y. Recovering metal values from spent lithium ion battery via a combination of reduction thermal treatment and facile acid leaching. ACS Sustain Chem Eng. 2018; 6: 10445−10453. https://doi.org/10.1021/acssuschemeng.8b01805
Othman EA, Van der Ham AGJ, Miedema H, Kersten SRA. Recovery of metals from spent lithium-ion batteries using ionic liquid [P8888][Oleate]. Sep Purif Technol. 2020; 252: 117435. https://doi.org/10.1016/j.seppur.2020.117435
Prabaharan G, Barik SP, Kumar N, Kumar L. Electrochemical process for electrode material of spent lithium ion batteries. Waste Manage. 2017; 68: 527−533. https://doi.org/10.1016/j.wasman.2017.07.007
Patnaik P, Padhy SK, Tripathy BC, Bhattacharya IN, Paramguru RK. Electrodeposition of cobalt from aqueous sulfate solutions in the presence of tetra ethyl ammonium bromide. Trans Nonferrous Met Soc of China 2015; 25: 2047−2053. https://doi.org/10.1016/S1003-6326(15)63814-6
Garcia EM, Santos JS, Pereira EC, Freitas MBJG. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique. J Power Sources 2008; 185: 549−553. https://doi.org/10.1016/j.jpowsour.2008.07.011
Bhuiyan MS, Taylor BJ, Paranthaman M, Thompson JR, Sinclair JW. Microstructure and magnetic properties of electrodeposited cobalt films. J Mater Sci. 2008; 43: 1644−1649. https://doi.org/10.1007/s10853-007-2383-2
Rafsanjani-Abbasi A, Rahimi E, Shalchian H, Vahdati-Khaki J, Babakhani A, Hosseinpour S, Davoodi A. Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel. Materials 2018; 12: 90. https://doi.org/10.3390/ma12010090
Rigsby MA, Spurlin TA, Reid JD. The Multi-Functional Role of Boric Acid in Cobalt Electrodeposition and Superfill. J Electrochem Soc. 2020; 167: 112507. https://doi.org/10.1149/1945-7111/aba640
Santos JS, Trivinho-Strixino F, Pereira EC. Investigation of Co(OH)2 formation during cobalt electrodeposition using a chemometric procedure. Surf Coat Technol. 2010; 205: 2585−2589. https://doi.org/10.1016/j.surfcoat.2010.10.005
Zhou J, Wang SF, Song XS. Electrodeposition of cobalt in double-membrane three-compartment electrolytic reactor. Trans Nonferrous Met Soc of China 2016; 26: 1706−1713. https://doi.org/10.1016/S1003-6326(16)64279-6
Zech N, Landolt D. The influence of boric acid and sulfate ions on the hydrogen formation in Ni-Fe plating electrolytes. Electrochim Acta 2000; 45: 3461−3471. https://doi.org/10.1016/S0013-4686(00)00415-1
Ho HY, Chen WB, Fu TY, Chen SJ. On the Electrodepositing of Cobalt Nanoparticles on ITO in the Presence of Boric Acid. IEEE Trans Magn. 2014; 50: 2100304. https://doi.org/10.1109/TMAG.2013.2277758
Altimari P, Schiavi PG, Rubino A, Pagnanelli F. Electrodeposition of cobalt nanoparticles: An analysis of the mechanisms behind the deviation from three-dimensional diffusion-control. J Electroanal Chem. 2019; 851: 113413. https://doi.org/10.1016/j.jelechem.2019.113413
Medić D, Milić S, Alagić S, Đorđević I, Dimitrijević S. Classification of spent Li-ion batteries based on ICP-OES/X-ray characterization of the cathode materials. Hem Ind. 2020; 74: 221−230. https://doi.org/10.2298/HEMIND200114012M
Medić VD, Sokić MD, Nujkić MM, Đorđievski SS, Milić SM, Alagić ČS, Antonijević MA. Cobalt extraction from spent lithium-ion battery cathode material using a sulfuric acid solution containing SO2. J Mater Cycles Waste Manage. 2023; 25: 1008–1018. https://doi.org/10.1007/s10163-022-01580-w
Nayl AA, Elkhashab RA, Badawy SM, El-Khateeb MA. Acid leaching of mixed spent Li-ion batteries. Arab J Chem. 2017; 10: S3632−S3639. https://doi.org/10.1016/j.arabjc.2014.04.001
Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manage. 2013; 33: 1890−1897. https://doi.org/10.1016/j.wasman.2013.05.008
Jiang F, Chen Y, Ju S, Zhu Q, Zhang L, Peng J, Wang X, Miller JD. Ultrasound-assisted Leaching of Cobalt and Lithium from Spent Lithium-ion Batteries. Ultrason Sonochem. 2018; 48: 88−95. https://doi.org/10.1016/j.ultsonch.2018.05.019
Gao W, Zhang X, Zheng X, Lin X, Cao H, Zhang Y, Sun ZHI. Lithium Carbonate, Recovery from Cathode Scrap of Spent Lithium-ion Battery - a Closed-loop Process. Environ Sci Technol. 2017; 51: 1662−1669. https://doi.org/10.1021/acs.est.6b03320
Zhu SG, He WZ, Li GM, Zhou X, Zhang XJ, Huang JW. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans Nonferrous Met Soc of China 2012; 22: 2274−2281. https://doi.org/10.1016/S1003-6326(11)61460-X
Kaskiala T. Determination of oxygen solubility in aqueous sulfuric acid media. Miner Eng. 2002; 15: 853−857. https://doi.org/10.1016/S0892-6875(02)00089-4
Ndalamo J, Mulaba-Bafubiandi AF, Mamba BB. UV/visible spectroscopic analysis of CO3+ and CO2+ during the dissolution of cobalt from mixed Co-Cu oxidized ores. Int J Min Met and Mater. 2011; 18: 260−269. https://doi.org/10.1007/s12613-011-0432-y
Šupicová M, Rozik R, Trnková L, Oriňáková, Gálová M. Influence of boric acid on the electrochemical deposition of Ni. J Solid State Electrochem. 2006; 10: 61−68. https://doi.org/10.1007/s10008-005-0656-8
Metikoš-Huković M, Babić R. Passivation and corrosion behaviours of cobalt and cobalt–chromium–molybdenum alloy. Corros Sci. 2007; 49: 3570–3579. https://doi.org/10.1016/j.corsci.2007.03.023
Avramović Lj, Maksimović VM, Baščarević Z, Ignjatović N, Bugarin M, Marković R, Nikolić ND. Influence of the Shape of Copper Powder Particles on the Crystal Structure and Some Decisive Characteristics of the Metal Powders. Metals 2019; 9: 56. https://doi.org/10.3390/met9010056