Adsorptive pretreatment of waste cooking oil using quicklime for fatty acid methyl esters synthesis Original scientific paper
Main Article Content
Abstract
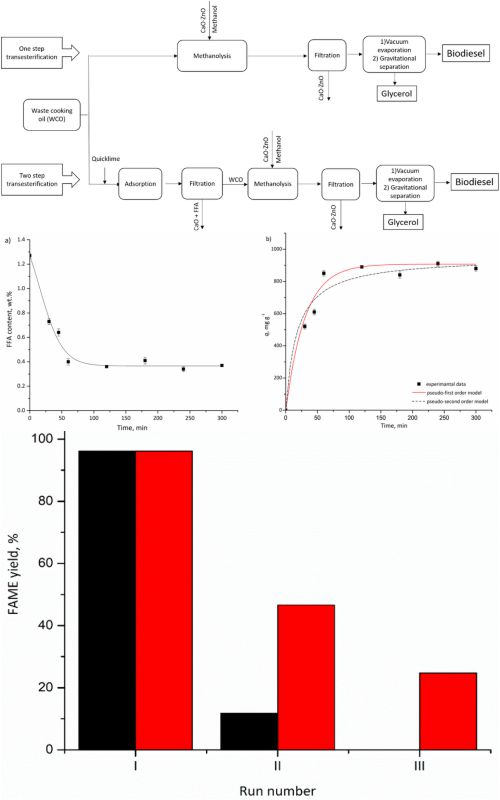
Synthesis of biodiesel from various plant oils is realized by the transesterification of triglycerides with methanol or by a reaction usually defined as methanolysis. The usage of low-quality oils, such as waste cooking oil (WCO), is followed by undesirable side reactions as a result of the increased content of free fatty acids (FFA), and water. The presence of FFA in WCO usually requires a pretreatment stage before subjecting it to methanolysis. In the present work, heterogeneously catalyzed methanolysis of WCO with and without pretreatment was investigated. Removal of FFA from WCO was conducted by using only quicklime or with the addition of a small amount of methanol (FFA to methanol = 1:3 molar ratio). The obtained results showed that pretreatment of WCO with quicklime at 30 °C after 1 h reduces the FFA content by 72 %, while the adsorption capacity was determined to be 910 mg g-1. The adsorptive pretreatment, as a simple operation, using low-cost quicklime under mild conditions, had a positive effect on the transesterification rate with CaO∙ZnO as a catalyst, enabling the achievement of over 96 % of biodiesel yield in only 15 min, compared to 1 h without the pretreatment. Furthermore, pretreated WCO allows an increase in repeated catalyst use and overall savings in the necessary amount of catalyst. The present study showed that quicklime is an economic, environmental-friendly, and sustainable material for FFA removal from WCO.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200135
References
Enweremadu CC, Mbarawa MM, Technical aspects of production and analysis of biodiesel from used cooking oil—A review. Renew Sust Energ Rev. 2009; 13: 2205–2224. https://doi.org/10.1016/j.rser.2009.06.007
Maddikeri GL, Pandit AB, Gogate PR, Intensification Approaches for Biodiesel Synthesis from Waste Cooking Oil: A Review. Ind Eng Chem Res. 2012; 51: 14610−14628. https://doi.org/10.1021/ie301675j
Yaakob Z, Mohammad M, Alherbawi M, Alam Z, Sopian K, Overview of the production of biodiesel from waste cooking oil. Renew Sust Energ Rev. 2013; 18: 184–193. https://doi.org/10.1016/j.rser.2012.10.016
Refaat AA, Different techniques for the production of biodiesel from waste vegetable oil. Int J Environ Sci Tech. 2010; 7(1): 183–213. https://doi.org/10.1007/BF03326130
Çanakçi M, Van Gerpen J, A pilot plant to produce biodiesel from high free fatty acid feedstocks Trans ASABE. 2003; 46: 945–954. https://doi.org/10.13031/2013.13949
Corro G, Tellez N, Jimenez T, Tapia A, Banuelos F, Vazquez-Cuchillo O, Biodiesel from waste frying oil. Two step process using acidified SiO2 for esterification step. Catal Today 2011; 166: 116–122. https://doi.org/10.1016/j.cattod.2010.09.011
Wang Y, Ou S, Liu P, Zhang Z, Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers Manag. 2007; 48: 184–188. https://doi.org/10.1016/j.enconman.2006.04.016
Cvengros J, Cvengrosova Z, Used Frying Oils and Fats and their Utilization in the Production of Methyl Esters of Higher Fatty Acids. Biomass Bioenerg. 2004; 27: 173–181. https://doi.org/10.1016/j.biombioe.2003.11.006
Díaz L, Brito A, FFA Adsorption from Waste Oils or Non-Edible Oils onto an Anion Exchange Resin as Alternative Method to Esterification Reaction Prior to Transesterification Reaction for Biodiesel Production. J Adv Chem Eng. 2014; 4: 105. http://dx.doi.org/10.4172/2090-4568.1000105
Demirbas A, Sari A, Isildak O, Adsorption thermodynamics of stearic acid onto bentonite. J Hazard Mater. 2006; 135: 226–231. https://doi.org/10.1016/j.jhazmat.2005.11.056
Sari A, Iþýldak Ö, Adsorption properties of stearic acid onto untreated kaolinite. Bull Chem Soc Ethiop. 2006; 20(2): 259–267. https://doi.org/10.1016/10.4314/bcse.v20i2.61410
Bayrak Y, Application of Langmuir isotherm to saturated fatty acid adsorption. Microporous and Mesoporous Mater. 2006; 87: 203–206. https://doi.org/10.1016/j.micromeso.2005.08.009
Sari A, Soylake M, Equilibrium and thermodynamic studies of stearic acid adsorption on Celtek clay. J Serb Chem Soc. 2007; 72(5): 485–494. https://doi.org/10.2298/JSC0705485S
Ilgen O, Adsorption of oleic acid from sunflower oil on Amberlyst A26 (OH). Fuel Process Technol. 2014; 118: 69–74. http://dx.doi.org/10.1016/j.fuproc.2013.08.012
Maddikeri GL, Pandit AB, Gogate PR, Adsorptive Removal of Saturated and Unsaturated Fatty Acids Using Ion-Exchange Resins. Ind Eng Chem Res. 2012; 51: 6869−6876. http://dx.doi.org/dx.doi.org/10.1021/ie3000562
Jamal Y, Boulanger BO, Separation of oleic acid from soybean oil using mixed-bed resins. J Chem Eng Data 2010; 55: 2405–2409. http://dx.doi.org/10.1021/je900829c
Ilgen O, Dulger HS, Removal of oleic acid from sunflower oil on zeolite 13X: Kinetics, equilibrium and thermodynamic studies. Ind Crops Prod. 2016; 81: 66–71. http://dx.doi.org/10.1016/j.indcrop.2015.11.050
Pereira MRDN, Salviano AB, de Medeiros TPV, Santos MRD, Cibaka TE, de Andrade MHC, de Oliveira Porto A, Lago RM. Ca(OH)2 nanoplates supported on activated carbon for the neutralization/removal of free fatty acids during biodiesel production. Fuel 2018; 221: 469–475. http://dx.doi.org/10.1016/j.fuel.2018.01.123
Nor Shafizah I, Irmawati R, Omar H, Yahaya M, Alia Aina A, Removal of free fatty acid (FFA) in crude palm oil (CPO) using potassium oxide/dolomite as an adsorbent: Optimization by Taguchi method. Food Chem. 2022; 373: 131668. https://doi.org/10.1016/j.foodchem.2021.131668
Díaz L, Mertes L, Brito A, Rodríguez KE, Valorization of energy crop shells as potential green adsorbents for free fatty acid removal from oils for biodiesel production. Biomass Convers Bioref. 2022; 12: 655–668. https://doi.org/10.1007/s13399-020-01089-y
Cano М., Sbargoud К., Allard Е., Larpent C, Magnetic separation of fatty acids with iron oxide nanoparticles and application to extractive deacidification of vegetable oils. Green Chem. 2012; 14: 1786–1795. https://doi.org/10.1039/c2gc35270b
Kalapathy U, Proctor A, New Method for Free Fatty Acid Reduction in Frying Oil Using Silicate Films Produced from Rice Hull Ash. JAOCS. 2000; 77(6): 593–598. https://doi.org/10.1007/s11746-000-0095-4
Clowutimon W, Kitchaiya P, Assawasaengrat P, Adsorption of Free Fatty Acid from Crude Palm Oil on Magnesium Silicate Derived From Rice Husk. Eng J. 15 2011; 15(3):15–25. https://doi.org/10.4186/ej.2011.15.3.15
Kim M, Yoon SH, Choi E, Gil B, Comparison of the adsorbent performance between rice hull ash and rice hull silica gel according to their structural differences. LWT-Food Sci Technol. 2008; 41: 701–706. https://doi.org/10.1016/j.lwt.2007.04.006
Topallar H, Bayrak Y, Investigation of Adsorption Isotherms of Myristic, Palmitic and Stearic Acids on Rice Hull Ash. Turk J Chem. 1999; 23: 193–198.
Adam F, Chua JH, The adsorption of palmitic acid on rice husk ash chemically modified with Al(III) ion using the sol–gel technique. J Colloid Interface Sci. 2004; 280: 55–61. https://doi.org/10.1016/j.jcis.2004.07.006
Bao Y, Zhou Q, Zhang M, Zhang H, Luan Q, Zhou W, Tang H, Huang F, Wet-spun nanoTiO2/chitosan nanocomposite fibers as efficient and retrievable absorbent for the removal of free fatty acids from edible oil. Carbohydr Polym 2019; 210: 119–126. https://doi.org/10.1016/j.carbpol.2019.01.035
Adewuyi A, Ogagbolo AI, Lau WJ, Oderinde RA, Synthesis of spinel ferrite and its role in the removal of free fatty acids from deteriorated vegetable oil. Chinese Journal of Chemical Engineering 2021; 40: 78–87. https://doi.org/10.1016/j.cjche.2020.08.054
Lukić I, Kesić Ž, Maksimović S, Zdujić M, Liu H, Krstić J, Skala D, Kinetics of sunflower and used vegetable oil methanolysis catalyzed by CaO·ZnO. Fuel 2013; 113: 367–78. https://doi.org/10.1016/j.fuel.2013.05.093
Kesić Ž, Lukić I, Brkić D, Rogan J, Zdujić M, Liu H, Skala D, Mechanochemical preparation and characterization of CaO·ZnO used as catalyst for biodiesel synthesis. Appl Catal A 2012; 427–428: 58–65. https://doi.org/10.1016/j.apcata.2012.03.032
Miladinović MR, Krstić JB, Tasić MB, Stamenković OS, Veljković VB, A kinetic study of quicklime-catalyzed sunflower oil methanolysis. Chem Eng Res Des. 2014; 92(9) 1740–1752. https://doi.org/10.1016/j.cherd.2013.11.023
Lukić I, Kesić Ž, Maksimović S, Zdujić M, Krstić J, Skala D. Kinetics of heterogeneous methanolysis of sunflower oil with CaO∙ZnO catalyst: Influence of different hydrodynamic conditions. Chem. Ind. Chem. Eng. Q. 2014; 20: 425–439. https://doi.org/10.2298/CICEQ130514025L
Qiu H, Lv L, Pan B, Zhang Q, Zhang W, Zhang Q, Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 2009; 10(5)716–724. https://doi.org/10.1631/jzus.A0820524
Song G, Zhu X, Chen R, Liao Q, Ding Y, Chen L, An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem Eng J. 2016; 283: 175–183.
Ho YS, McKay G, Pseudo-second order model for sorption processes. Process Biochem. 1999: 34(5): 451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Filopoulou A, Vlachou S, Boyatzis SC, Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage. Molecules 2021; 26(19): 6005. https://doi.org/10.3390/molecules26196005
Lu Y, Miller JD, Carboxyl Stretching Vibrations of Spontaneously Adsorbed and LB-Transferred Calcium Carboxylates as Determined by FTIR Internal Reflection Spectroscopy. J Colloid Interface Sci. 2002; 256: 41–52. https://doi.org/doi:10.1006/jcis.2001.8112
Zul NA, Ganesan S, Hamidon TS, Oh W-D, Hussin MH, A review on the utilization of calcium oxide as a base catalyst in biodiesel production. J Environ Chem Eng 2021: 9(4): https://doi.org/10.1016/j.jece.2021.105741
Lin Y-C, Amesho KTT, Chen C-E, Cheng P-C, Chou F-C, A cleaner process for green biodiesel synthesis from waste cooking oil using recycled waste oyster shells as a sustainable base heterogeneous catalyst under the microwave heating system. Sustain Chem Pharm 2020; 17: 100310 https://doi.org/10.1016/j.scp.2020.100310
Aghel B, Mohadesi M, Ansari A, Maleki M, Pilot-scale production of biodiesel from waste cooking oil using kettle limescale as a heterogeneous catalyst. Renew Energy 2019; 142: 207–214. https://doi.org/10.1016/j.renene.2019.04.100
Yusuff AS, Gbadamosi AI, Atray N, Development of a zeolite supported CaO derived from chicken eggshell as active base catalyst for used cooking oil biodiesel production. Renew Energy 2022; 197: 1151–1162. https://doi.org/10.1016/j.renene.2022.08.032
Borah MJ, Das A, Das V, Bhuyan N, Deka D, Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019; 242: 345–354. https://doi.org/10.1016/j.fuel.2019.01.060.
Torkzaban S, Feyzi M, Norouzi L, A novel robust CaO/ZnFe2O4 hollow magnetic microspheres heterogenous catalyst for synthesis biodiesel from waste frying sunflower oil, Renew Energy 2022; 200: 996–1007. https://doi.org/10.1016/j.renene.2022.09.077.
Foroutan R, Mohammadi R, Razeghi J, Ramavandi B, Biodiesel production from edible oils using algal biochar/CaO/K2CO3 as a heterogeneous and recyclable catalyst. Renew Energy 2021; 168: 1207–1216. https://doi.org/10.1016/j.renene.2020.12.094
López Granados M, Martín Alonso D, Sádaba I, Mariscal R, Ocón P, Leaching and homogeneous contribution in liquid phase reaction catalysed by solids: the case of triglycerides methanolysis using CaO. Appl Catal B 2009; 89: 265–272. https://doi.org/10.1016/j.apcatb.2009.02.014
Pavlović SM, Marinković DM, Kostić MD, Janković-Častvan IM, Mojović LjV, Stanković MV, Veljković VB, A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production. Fuel 2020; 267: 117171. https://doi.org/10.1016/j.fuel.2020.117171.
Kostić MD, Bazargan A, Stamenković OS, Veljković VB, McKay G, Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel 2016; 163: 304–313. 10.1016/j.fuel.2015.09.042.
Acosta PI, Campedelli RR, Correa EL, Bazani HAG, Nishida EN, Souza BS, Mora JR, Efficient production of biodiesel by using a highly active calcium oxide prepared in presence of pectin as heterogeneous catalyst. Fuel 2020; 271: 117651. https://doi.org/10.1016/j.fuel.2020.117651.
López Granados M, Martín Alonso D, Sádaba I, Mariscal R, Ocón P, Surface chemical promotion of Ca oxide catalysts in biodiesel production reaction by the addition of monoglycerides, diglycerides and glycerol. J Catal 2010; 276: 229–236. https://doi.org/10.1016/j.jcat.2010.09.016