PROCESS MODELING AND KINETIC ESTIMATION FOR DESULFURIZATION OF DIESEL FUEL USING NANO - ZnO/Al2O3
Original scientific paper
DOI:
https://doi.org/10.2298/CICEQ230208020HKeywords:
gamma alumin, model, nano-catalyst, optimization, sulfur, zinc oxideAbstract
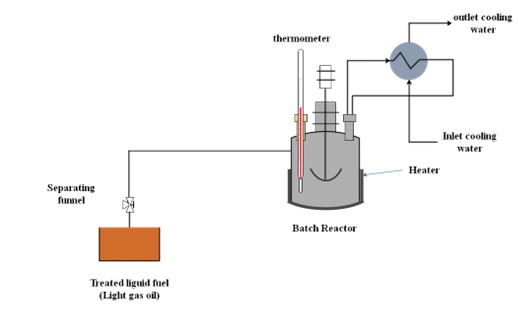
In the present paper, a gamma alumina (γ-Al2O3) loaded zinc oxide (ZnO) nano-catalyst (ZnO/γ-Al2O3) has been synthesized and used to accelerate the removal of sulfur compounds from light gas oil by oxidative desulfurization (ODS) process. The synthesized nano-catalysts have been characterized by atomic force microscopy (AFM) and Brunauer-Emmett-Teller (BET). The ODS process has been conducted in a batch reactor at various reaction temperatures and batch times varying between 30 to 90 °C and 20 to 80 min, respectively. DBT removal was highest (93.781%) while using synthesized nano-catalyst (9% ZnO/γ-Al2O3) at 90°C and 80 min reaction time. Based on the obtained experimental data, a new mathematical modeling technique was performed for the ODS operation under mild experimental conditions to evaluate the most appropriate kinetic variables for the newly synthesized nano-catalysts. Simulation results indicate a good match with experimental observations with less than 5% absolute average error for all runs. The optimization procedure of the process condition displays that > 98% DBT could be eliminated within 200 min, at 87 °C, in the existence of synthesized nano-catalyst (9% ZnO/γ-Al2O3).
References
S.A. Jafar, A.T. Nawaf, J.I. Humadi, Mater. Today: Proc. 42 (2021) 1777—1783. https://doi.org/10.1016/j.matpr.2020.11.821.
J.I. Humadi, S.A. Gheni, S.M.R. Ahmed, G.H. Abdullah, A.N. Phan, A.P. Harvey, Process Saf. Environ. Prot. 152 (2021) 178—187. https://doi.org/10.1016/j.psep.2021.05.028.
G.S. Ahmed, J.I. Humadi, A.A. Aabid, Iraqi. J. Chem. Pet. Eng. 22 (2021) 11—17. https://doi.org/10.31699/IJCPE.2021.3.2.
A.T. Albayrak, A. Tavman, Ultrason. Sonochem. 83 (2022) 105845. https://doi.org/10.1016/j.ultsonch.2021.105845.
A. Akopyan, E. Eseva, P. Polikarpova, A. Kedalo, A. Vutolkina, A. Glotov, Molecules 25 (2020) 536. https://doi.org/10.3390/molecules25030536.
H. Zhao, G.A. Baker, Front. Chem. Sci. Eng. 9 (2015) 262—279. https://doi.org/10.1007/s11705-015-1528-0.
N.P. Radhika, R. Selvin, R. Kakkar, A. Umar, Arabian J. Chem. 12 (2019) 4550—4578. https://doi.org/10.1016/j.arabjc.2016.07.007.
P. Polikarpova, A. Akopyan, A. Shigapova, A. Glotov, A. Anisimov, E. Karakhanov, Energy Fuels 32 (2018) 10898—10903. https://doi.org/10.1021/acs.energyfuels.8b02583.
S. Subhan, A.U. Rahman, M. Yaseen, H.U. Rashid, M. Ishaq, M. Sahibzada, Z. Tong, Fuel 237 (2019) 793—805. https://doi.org/10.1016/j.fuel.2018.10.067.
Z. Ismagilov, S. Yashnik, M. Kerzhentsev, V. Parmon, A. Bourane, F. M. Al-Shahrani, A. A. Hajji, O. R. Koseoglu, Catal. Rev.: Sci. Eng. 53 (2011) 199—255. https://doi.org/10.1080/01614940.2011.596426.
J. I. Humadi, S. A. Gheni, S. M. Ahmed, A. Harvey, RSC Adv. 12 (2022) 14385—14396. https://doi.org/10.1039/D2RA01663J.
J. I. Humadi, A.T. Nawaf, A.T. Jarullah, M.A. Ahmed, S.A. Hameed, I. M. Mujtaba, Chem. Eng. Res. Des. 190 (2023) 634—650. https://doi.org/10.1016/j.cherd.2022.12.043.
J.I. Humadi, Y.S. Issa, D. Y. Aqar, M. A. Ahmed, H.H. Ali Alak, I.M. Mujtaba, Int. J. Chem. React. Eng. 21(6) (2023) 727—741. https://doi.org/10.1515/ijcre-2022-0046.
M.I. Fathi, J.I. Humadi, Q.A. Mahmood, A.T. Nawaf, R.S. Ayoub, AIP Conf. Proc. 2660 (2022) 020026 https://doi.org/10.1063/5.0109089.
A.A. Aabid, J.I. Humadi, G.S. Ahmed, A.T. Jarullah, M.A. Ahmed, W.S. Abdullah, Appl. Sci. Eng. Prog. (2023). https://doi.org/10.14416/j.asep.2023.02.007.
A.T. Nawaf, A.T. Jarullah, L.T. Abdulateef, Bull. Chem. React. Eng. Catal. 14 (2019) 79—92. https://doi.org/10.9767/bcrec.14.1.2507.79-92.
P. Huang, G. Luo, L. Kang, M. Zhu, B. Dai, RSC Adv. 7 (2017) 4681—4687. https://doi.org/10.1039/C6RA26587A.
B. Saha, S. Kumar, S. Sengupta, Chem. Eng. Sci. 199 (2019) 332—341. https://doi.org/10.1016/j.ces.2018.12.063.
S.A. Ghazwan, A.T. Jarullah, B. Al-Tabbakh, I.M. Mujtaba, J. Cleaner Prod. 257 (2020) 120436. https://doi.org/10.1016/j.jclepro.2020.120436.
A.T. Jarullah, K. Sarmad, B. Al-Tabbakh, I.M. Mujtaba, Chem. Prod. Process Model. 17(3) (2022) 213—233. https://doi.org/10.1515/cppm-2020-0097.
A.T. Nawaf, H.H. Hamed, S.A. Hameed, A.T. Jarullah, I.M. Mujtaba, Chem. Eng. Sci. 232 (2021) 116384. https://doi.org/10.1016/j.ces.2020.116384.
A.T. Nawaf, A.T. Jarullah, Sh. A. Hameed, I.M. Mujtaba, Chem. Prod. Process Model. 16(3) (2021) 229—249 (2021), https://doi.org/10.1515/cppm-2020-0107.
A.T. Jarullah, S.K. Aldulaimi, B.A. Al-Tabbakh, I.M. Mujtaba, Chem. Eng. Res. Des. 160 (2020) 405—416. https://doi.org/10.1016/j.cherd.2020.05.015.
K.I. Hamad, J.I. Humadi, Y.S. Issa, S.A. Gheni, M.A. Ahmed, A.A. Hassan, Cleaner Eng. Technol. 11 (2022). 100570. https://doi.org/10.1016/j.clet.2022.100570.
A.T. Jarullah, I.M. Mujtaba, A.S. Wood, Fuel 90 (2011) 2165—2181. https://doi.org/10.1016/j.fuel.2011.01.025.
N. Ghorbani, G. Moradi Chin. J. Chem. Eng. 27 (2019) 2759—2770. https://doi.org/10.1016/j.cjche.2019.01.037.
S. A. Barham, L. O. Hamasalih, K. H. H. Aziz, K. M. Omer, & I. Shafiq, Proc. 10 (2022) 2327. https://doi.org/10.3390/pr10112327.
B. S. Ahmed, L. O. Hamasalih, K. H. H. Aziz, Y. M. Salih, F. S. Mustafa, & K. M. Omer, Sep. 10 (2023) 206. https://doi.org/10.3390/separations10030206.
J. I. Humadi, S. A. Jafar, N. S. Ali, M. A. Ahmed, M. J. Mzeed, R. J. Al-Salhi, & T. M. Albayati, Sci. Rep. 13 (2023) 9931. https://doi.org/10.1038/s41598-023-37188-9.
G. H. A. Razzaq, M. A. Shihab, J. I. Humadi, K. K. Saxena, C. Prakash, & L. I. Saeed, Mater. Today: Proc. (2023). https://doi.org/10 .1016/j.matpr.2023.05.432.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Jasim I. Humadi, Muayad A. Shihab, Ghazwan S. Ahmed, Mustafa A Ahmed, Zeyad A. Abdullah, Shankar Sehgal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.





