CO2 MITIGATION STUDIES IN PACKED ABSORPTION COLUMN USING IRON OXIDE NANOFLUID
Scientific paper
DOI:
https://doi.org/10.2298/CICEQ210510023SKeywords:
CO2 removal efficiency, interfacial area, mass transfer coefficient, nano fluid, packed column, absorptionAbstract
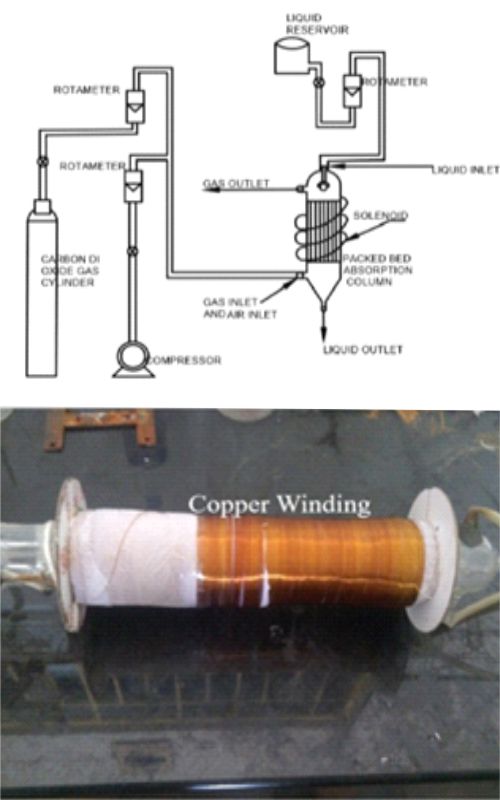
The challenging task in our ecosystem is to reduce acidic gas emissions to some extent. Many gases are emitted from the industries like H2S, CO, CO2, SO2, NO, and NO2 as exhaust gases. Among these gases, CO2, NO2, and SO2 are acidic, which results in adverse effects on humans, animals, and plants. The increase in the emission of CO2 gases from both anthropogenic and industrial sources resulted in CO2 mitigation studies. CO2 absorption studies were carried out using iron oxide nanofluid with the novel structured packed absorption column. Iron oxide nanoparticles were synthesized and characterized using XRD, SEM, and TEM analysis. Ammonia is used as an absorbent along with iron oxide nanofluid of three different concentrations (0.0001 w/v%, 0.001 w/v%, and 0.0015 w/v%). It was found that the iron oxide nanofluid of 0.0015 w/v% showed an improved % CO2 removal efficiency. This enhanced % CO2 removal efficiency was due to the increased interfacial area of the ameliorated contact between the liquid and gas phases. In addition, the magnetic field was introduced along with the packed column, which increased CO2 removal efficiency by 1.5%.
References
A. Aroonwilas, Ind. Eng. Chem. Res. 43 (2004) 2228—2237. https://doi.org/10.1021/ie0306067.
A. Aroonwilas, P. Tontiwachwuthikul, Chem. Eng. Sci. 55 (2000) 3651—3663. https://doi.org/10.1016/S0009-2509(00)00035-X.
W.M.Budzianowski, R.Miller, Recent Pat. Mech. Eng. 2 (2009) 228—239. https://doi.org/10.2174/1874477X10902030228.
T.W. Chien, H. Chu, H.T. Hsueh, J. Environ. Eng. 129 (2003) 967—974. https://doi.org/10.1061/(ASCE)0733-9372(2003)129:11(967).
F. Zhang, C.-G. Fang, Y.-T. Wu, Y.-T. Wang, A.-M. Li, Z.-B. Zhang, Chem. Eng. J. 160 (2010) 691—697. https://doi.org/10.1016/j.cej.2010.04.013.
H. Monnier, L. Falk, Chem. Eng. Sci. 66 (2011) 2475—2490. https://doi.org/10.1016/j.ces.2011.01.016.
H. Monnier, L. Falk, N. Mhiri, Chem. Eng. Process. 49 (2010) 953—957. https://doi.org/10.1016/j.cep.2010.05.001.
J. Salimi, F. Salimi, Rev. Mex. Ing. Quim. 15 (2016) 185—192. http://www.rmiq.org/ojs311/index.php/rmiq/article/view/1106/413.
J. Salimi, F. Salimi, Heat Mass Transfer 51 (2015) 621—629. https://doi.org/10.1007/s00231-014-1439-5.
A.O. Lawal, R.O. Idem, Ind. Eng. Chem. Res. 45 (2006) 2601—2607. https://doi.org//10.1021/ie050560c.
R. Notz, N. Asprion, I. Clausen, H. Hasse, Chem. Eng. Res. Des. 85 (2007) 510—515. https://doi.org/10.1205/cherd06085.
P. Oinas, G. Wild, N. Midoux, H. Haario, Chem. Eng. Process. 34 (1995) 503—513. https://doi.org/10.1016/0255-2701(95)00454-8.
O. Lawal, A. Bello, R. Idem, Ind. Eng. Chem. Res. 44 (2005) 1874—1879. https://doi.org/10.1021/ie049261y.
Z. Niu, Y. Guo, Q. Zeng, W. Lin, Ind. Eng. Chem. Res. 51 (2012) 5309—5319. https://doi.org/10.1021/ie2030536.
Q. Zeng, W. Lin, Y. Guo, Z. Niu, Fuel Process. Technol. 108 (2013) 76—81. https://doi.org/10.1016/j.fuproc.2012.05.005.
P.P. Selvi, R. Baskar, P.S. Nair, J. Adv. Chem. 13 (2017) 6520—6523. https://doi.org/10.24297/jac.v13i10.5789.
P.P. Selvi, R. Baskar, J. Chem. Soc. Pak. 41 (2019) 820—824. https://jcsp.org.pk/PublishedVersion/3b17aa02-80a4-466d- aaa9- cff35adc7448Manuscript%20no%2010,%20Final%20Gally%20Proof%20of%2011943%20(Pongayi%20Ponnusamy%20Selvi).pdf.
P.P. Selvi, R. Baskar, Chem. Ind. Chem. Eng. Q. 26 (2020) 321—328. https://doi.org/10.2298/CICEQ181225008S.
S.-S. Ashrafmansouri, M.N. Esfahany, Inter. J. Therm. Sci. 82 (2014) 84—99. https://doi.org/10.1016/j.ijthermalsci.2014.03.017.
W. Yu, H. Xie, J. Nano Mater. 2012 (2011) ID 435873. https://doi.org/10.1155/2012/435873.
W. Hao, E. Bjorkman, M. Lilliestrale, N. Hedin, Chem. Sustainability 7 (2014) 875—882. https://doi.org/10.1002/cssc.201300912.
W. Yuan, B. Li B, L. Li, Appl. Surf. Sci.257 (2011) 10183—10187. https://doi.org/10.1016/j.apsusc.2011.07.015.
Z. Zhang, W. Zhang, X. Chen, Q. Xia, Z. Li, Sep. Sci. Technol. 45 (2010) 710—719. https://doi.org/10.1080/01496390903571192.
M. M. Tun, D. Juchelková, Environ. Eng. Res. 24 (2019) 618—629. http://dx.doi.org/10.4491/eer.2018.327.
Z. Samadi, M Haghshenasfard, A Moheb, Chem. Eng. Technol. 37 (2014) 462—470. https://doi.org/10.1002/ceat.201300339.
M. Ansaripour, M Haghshenasfard, A Moheb, Chem. Eng. Technol. 41 (2018) 367—378. https://doi.org/10.1002/ceat.201700182.
M.Khani, M. Haghshenasfard, N. Etesami, M.R. Talaei, J. Mol. Liq. 334 (2021) 116078. https://doi.org/10.1016/j.molliq.2021.116078.
Downloads
Published
Issue
Section
License
Copyright (c) 2021 Pongayi Ponnusamy Selvi, Rajoo Baskar

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.



