OBTAINING XYLITOL BY HYDROLYSIS-HYDROGENATION OF LIQUORS DERIVED FROM SUGARCANE BAGASSE
Scientific paper
DOI:
https://doi.org/10.2298/CICEQ210721012CKeywords:
Sugarcane bagasse, Hydrolysis, Hydrogenation, Xylose, XylitolAbstract
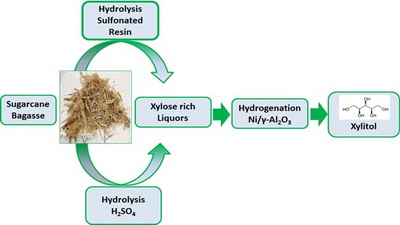
This work presents the study of heterogeneous catalysis of sugarcane bagasse hydrothermal treatment spent liquors using a sulfonated resin. Besides, results were compared with those obtained by a conventional route using sulfuric acid as a homogeneous catalyst. Heterogeneous catalysis is suitable for the hydrolysis of sugarcane bagasse hydrothermal liquors under mild conditions (100 °C and 6 h). The obtained maximum xylose yield was 82% due to furfural formation, which causes a xylose selectivity drop. The hydrogenation of this xylose-rich liquor at 100 °C and 3 MPa of hydrogen pressure employing a supported Ni/γ-Al2O3 produced the total conversion of xylose with a selectivity towards xylitol of 100% by using a catalyst to xylose mass ratio of 0.5. Heterogeneous catalysis in a two-step route (hydrolysis and hydrogenation) constitutes an outstanding alternative to producing xylitol from sugarcane bagasse hydrothermal spent liquors since materials can be easily separated and reused in several reaction cycles.
References
M. FitzPatrick, P. Champagne, M. F. Cunningham, R. A. Whitney, Bioresour. Technol. 101 (2010) 8915—8922. https://doi.org/10.1016/j.biortech.2010.06.125
W.E. Mabee, P.N. McFarlane, J.N. Saddler, Biomass Bioenergy 35 (2011) 4519—4529. https://doi.org/10.1016/j.biombioe.2011.06.026
M.E. Vallejos, F.E. Felissia, M. C. Area, Bioresources 12 (2017) 2058—2080. https://bioresources.cnr.ncsu.edu/wp-content/uploads/2017/02/BioRes_12_1_2058_REVIEW_Vallejos_FA_Hydrothermal_Treatm_Agro_Forest_Indust_Waste_Value_10459.pdf
Food and Agriculture Organization (FAO). Statistics Division of the FAO (FAOSTAT). http://www.fao.org/faostat/en/#data/QC, [accessed 19 February 2022].
N. Clauser, S. Gutiérrez, M.C. Area, F.E. Felissia, M.E. Vallejos. J. Renew. Mater. 6 (2018) 139—151. http://dx.doi.org/10.7569/JRM.2017.634145
H. Boussarsar, B. Rogé, M. Mathlouthi, Bioresour. Technol. 100 (2009) 6537—6542. https://doi.org/10.1016/j.biortech.2009.07.019
M. Fatih Demirbas, Appl. Energy 86 (2009) 151—161. https://doi.org/10.1016/j.biortech.2009.07.019
D.J. Hayes, Catal. Today 145 (2009) 138—151. https://doi.org/10.1016/j.cattod.2008.04.017
M.E. Vallejos, F.E. Felissia, J. Kruyeniski, M.C. Area, Ind. Crops Prod. 67 (2015) 1—6. https://doi.org/10.1016/j.indcrop.2014.12.058
L. da Costa Sousa, S. Chundawat, V. Balan, B. E. Dale, Curr. Opin. Biotechnol. 20 (2009) 339—347. https://doi.org/10.1016/j.copbio.2009.05.003
Y. Delgado Arcaño, O.D. Valmaña Garcia, D. Mandelli, W.A. Carvalho, L.A. Magalhães Pontes, Catal. Today 344 (2020) 2—14. https://doi.org/10.1016/j.cattod.2018.07.060
K. Nakajima, M. Hará, ACS Catal. 2 (2012) 1296—1304. https://doi.org/10.1021/cs300103k
S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi, M. Hara, J. Am. Chem. Soc. 130 (2008) 12787—12793. https://doi.org/10.1021/ja803983h
M.E. Vallejos, M. Chade, E. Beda Mereles, D.I. Bengoechea, J.G. Brizuela, F.E. Felissia, M.C. Area, Ind. Crops Prod. 91 (2016) 161—169. https://doi.org/10.1016/j.indcrop.2016.07.007
P.L. Dhepe, R. Sahu, Green Chem. 12 (2010) 2153—2156. https://doi.org/10.1039/C004128A
Y. Jiang, X. Li, X. Wang, L. Meng, H. Wang, G. Peng, X. Wang, X. Mu, Green Chem. 14 (2012) 2162—2167. https://doi.org/10.1039/C2GC35306G
M. Kitano, D. Yamaguchi, S. Suganuma, K. Nakajima, H. Kato, S. Hayashi, M. Hara, Langmuir 25 (2009) 5068—5075. https://doi.org/10.1021/la8040506
B.T. Kusema, G. Hilmann, P. Mäki-Arvela, S. Willför, B. Holmbom, T. Salmi, D.Y. Murzin, Catal. Lett. 141 (2011) 408—412. https://doi.org/10.1007/s10562-010-0530-x
Y. Ogaki, Y. Shinozuka, T. Hara, N. Ichikuni, S. Shimazu, Catal. Today 164 (2011) 415—418. https://doi.org/10.1016/j.cattod.2010.11.002
M. Okamura, A. Takagaki, M. Toda, J.N. Kondo, K. Domen, T. Tatsumi, M. Hara, S. Hayashi, Chem. Mater. 18 (2006) 3039—3045. https://doi.org/10.1021/cm0605623
A. Onda, T. Ochi, K. Yanagisawa, Green Chem. 10 (2008) 1033—1037. https://doi.org/10.1039/B808471H
R. Sahu, P.L. Dhepe, ChemSusChem 5 (2012) 751—761. https://doi.org/10.1002/cssc.201100448
L. Zhou, M. Shi, Q. Cai, L. Wu, X. Hu, X. Yang, C. Chen, J. Xu, Microporous Mesoporous Mater. 169 (2013) 54—59. https://doi.org/10.1016/j.micromeso.2012.10.003
P.D. Cará, M. Pagliaro, A. Elmekawy, D.R. Brown, P. Verschuren, N.R. Shiju, G. Rothenberg, Catal. Sci. Technol. 3 (2013) 2057—2061. https://doi.org/10.1039/C3CY20838A
R. Ormsby, J. R. Kastner, J. Miller, Catal. Today 190 (2012) 89—97. https://doi.org/10.1016/j.cattod.2012.02.050
J.P. Mikkola, R. Sjöholm, T. Salmi, P. Mäki-Arvela, Catal. Today 48 (1999) 73—81. https://doi.org/10.1016/S0920-5861(98)00360-5
J. Wisniak, M. Hershkowitz, R. Leibowitz, S. Stein, Ind. Eng. Chem. Prod. Res. Dev. 13 (1974) 75—79. https://doi.org/10.1021/i360049a015
M. Yadav, D.K. Mishra, J. Hwang, Appl. Catal., A 425 (2012) 110—116. https://doi.org/10.1016/j.apcata.2012.03.007
J. Lee, Y. Xub, G.W. Huber, Appl. Catal., B 140 (2013) 98—107. https://doi.org/10.1016/j.apcatb.2013.03.031
J. Wisniak, M. Hershkowitz, S. Stein, Ind. Eng. Chem. Prod. Res. Dev. 13 (1974) 232—236. https://doi.org/10.1021/i360052a004
F. Devred, A.H. Gieskea, N. Adkins, U. Dahlborg, C.M. Bao, M. Calvo-Dahlborg, J.W. Bakker, B.E. Nieuwenhuys, Appl. Catal., A 356 (2009) 154—161. https://doi.org/10.1016/j.apcata.2008.12.039
A. Gervasini, J. Fenyvesi, A. Auroux, Catal. Lett. 43 (1997) 219—228. https://doi.org/10.1023/A:1018979731407
G. Busca, Catal. Today 226 (2014) 2—13. https://doi.org/10.1016/j.cattod.2013.08.003
D.S. Brands, U.A. Sai, E.K. Poels, A. Bliek, J. Catal. 186 (1999) 169—180. https://doi.org/10.1006/jcat.1999.2553
L.S. Carvalho, C.L. Pieck, M.L. Rangel, N.S. Figoli, C.R. Vera, J.M. Parera, Appl. Catal., A 269 (2004) 105—116. https://doi.org/10.1016/j.apcata.2004.04.006
A. Yamaguchi, O. Sato, N. Mimura, M. Shirai, Catal. Today 265 (2016) 199—202. https://doi.org/10.1016/j.cattod.2015.08.026
L.S. Ribeiro, J.J. Delgado, J.J. de Melo Órfão, M.F.R. Pereira, RSC Adv. 6 (2016) 95320—95327. https://doi.org/10.1039/C6RA19666G
L. Venkateswar Rao, J.K. Goli, J. Gentela, S. Koti, Bioresour. Technol. 213 (2016), 299—310. https://doi.org/10.1016/j.biortech.2016.04.092
O.A. Ogunyewo, P. Upadhyay, G.H. Rajacharya, O. E. Okereke, L. Faas, L.D. Gómez, S. J. McQueen‑Mason, S. S. Yazdani, Biotechnol. Biofuels. 14 (2021), 1—17. https://doaj.org/article/aeeb042f69924f6787414bdcf3e672ab
A.F. Hernández-Pérez, A.C. Chaves-Villamil, P.V. de Arruda, J.C. dos Santos, M. das G. de A. Felipe, Waste Biomass Valorization 11 (2020), 4215—4224. https://doi.org/10.1007/s12649-019-00742-6
M.S.S, Reshamwala, A.M. Lali, Biotechnol. Prog. 36 (2020), e2972. https://doi.org/10.1002/btpr.2972
G. Guirimand, K. Inokuma, T. Bamba, M. Matsuda, K. Morita, K. Sasaki, C. Ogino, J.-G. Berrin, T. Hasunuma, A. Kondo, Green Chem. 21 (2019), 1795—1808. https://doi.org/10.1039/C8GC03864C
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.





