ADSORPTIVE REMOVAL OF CRYSTAL VIOLET DYE FROM AQUEOUS SOLUTION ONTO COCONUT COIR
Scientific paper
DOI:
https://doi.org/10.2298/CICEQ211203009AKeywords:
Coconut coir, sodium chlorite, crystal violet, adsorption kinetics, adsorption mechanism, density functional theoryAbstract
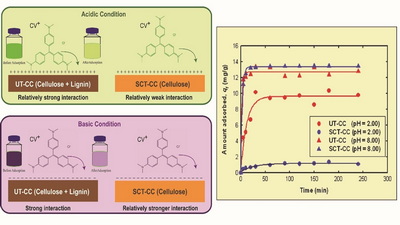
The untreated and sodium chlorite-treated coconut coir was implemented to remove crystal violet (CV) dye from an aqueous solution by batch adsorption experiments. The adsorption capacity, equilibrium time, and adsorption kinetics of CV on both adsorbents were regulated by the pH of the dye solution. High pH favors the comparative adsorption capacity for both adsorbents. In contrast, the untreated coconut coir (UT-CC) shows higher adsorption efficiency (9.61 mg g-1) than sodium chlorite-treated coconut coir (SCT-CC) at low pH. At lower pH (2.00), the equilibrium was established within 60 min by both adsorbents. However, the quick attainment of the equilibrium (30 min) was observed using both the adsorbents at higher pH (8.00). The isotherm data for both the adsorbents was found to have better agreement with the Freundlich than the Langmuir model at pH 8.00. The kinetic data was well-fitted with Ho’s pseudo-second-order model. Both adsorbents were characterized by FTIR and SEM to get evidence for the proposed adsorption mechanism. Density functional theory (DFT) also supports this result which illustrates the adsorption of CV on lignin of CC with the adsorption energy -51.16 kJ/mol at the B3LYP/6-31(d,p) level of theory.
References
J.H. Weisburger, Mutat. Res. 506-507 (2002) 9—20. https://doi.org/10.1016/S0027-5107(02)00147-1
R.O. Alves de Lima, A. P. Bazo, D.M.F. Salvadori, C. M. Rech, O. Danielle de Palma, U. Gisela de Aragao, Mut. Res. 626 (2007) 53—60. https://doi.org/10.1016/j.mrgentox.2006.08.002
N. Mathur, P. Bhatnagar, P. Sharma, Univ. J. Environ. Res. Technol. 2(2) (2012) 1—18. https://www.environmentaljournal.org/2-2/ujert-2-2-1.pdf
B. Lellis, C.Z. Fávaro-Polonio, J.A. Pamphile, J.C. Polonio, Biotechnol. Res. Innov. 3(2) (2019) 275—290. https://doi.org/10.1016/j.biori.2019.09.001
M .Sudha, A. Saranya, G. Selvakumar, N. Sivakumar, Int. J. Curr. Microbiol. App. Sci. 3(2) (2014) 670—690. https://www.ijcmas.com/vol-3-2/M.Sudha,%20et%20al.pdf
S. P. Upadhyay, J. Environ. Res. Dev. 3(2) (2008) 490—494.http://www.jerad.org/ppapers/V003N002/V003N002P0490.pdf
A. Gurses, C. Dogar, M. Yalcin, M. Acikyildiz, R. Bayrak, S. Karaca, J. Hazard. Mater. 131(6) (2006) 217—228. https://doi.org/10.1016/j.jhazmat.2005.09.036
C. Allegre, P. Moulin, M. Maisseu, F. Charbit, J. Membr. Sci. 269(4) (2006) 15—34. https://doi.org/10.1016/j.memsci.2005.06.014
K. Kadirvelu, K. Thamaraiselvi, C. Namasivayam, Bioresour. Technol. 76 (2001) 63—65. https://doi.org/10.1016/S0960-8524(00)00072-9
J.A. Ramsay, T. Nguyen, Biotech. Lett. 24(21) (2002) 1757—1761. https://doi.org/10.1023/A:1020644817514
K.B. Hu, Y.L. Wang, C.R. Li, Y.Q. Zheng, Acta Sci. Circumstantiae 30(11) (2010) 2174—2183. http://caod.oriprobe.com/articles/25753718/Fractal_characteristics_of_adsorption_of_direct_dye_compounds_onto_mod.htm
E. Bascetin, G. Atun, J. Chem. Eng. Data 55 (2010) 783—788. https://doi.org/10.1021/je9004678
M.L. Zanota, N. Heymans, F. Gilles, B.L. Su, M. Frer, G.D. Weireld, J. Chem. Eng. Data 55 (2010) 448—458. https://doi.org/10.1021/je900539m
S. Madhavakrishnan, K. Manickavasagam, R. Vasanthakumar, K. Rasappan, R. Mohanraj, S. Pattabhi, e-J. Chem. 6(4) (2009) 1109—1116. https://doi.org/10.1155/2009/764197
A. Adak, M. Bandyopadhyay, A. Pal, Sep. Purif. Technol. 44(2) (2005) 139—144. https://doi.org/10.1016/j.seppur.2005.01.002
C.D. Lin, C.T. Chen, Anim. Feed Sci. Technol. 54 (1995) 217—226. https://doi.org/10.1016/0377-8401(94)00760-7
C.L. Hall, P.B. Hamilton, Poult. Sci. 6 (1982) 62—66. https://doi.org/10.3382/ps.0610062
R.L. Berrios, J.L. Arbiser, Dermatol. Clin. 29(1) (2011) 69—73. https://doi.org/10.1016/j.det.2010.08.009
A. Pona, E.Y. Quan, A. Cline, S.R. Feldman, Dermatol Online J. 26(5) (2020) 13030.
https://doi.org/10.5070/D3265048772
P. Das, S. Chakraborty, S. Chowdhury, Arch. Environ. Sci. 6 (2012) 57—61. https://aes.asia.edu.tw/Issues/AES2012/SahaPD2012-1.pdf
A. Mittal, J. Mittal, A. Malviya, D. Kaur, V.K. Gupta, J. Colloid Interface Sci. 343 (2009) 463—473. https://doi.org/10.1016/j.jcis.2009.11.060
Y. Lin, X. He, G. Han, Q. Tian, W. Hu, J. Environ. Sci. 23(12) (2011) 2055—2062. https://doi.org/10.1016/S1001-0742(10)60643-2
U.J. Etim, E. Inam, S.A. Umoren, U.M. Eduok, Int. J. Environ. Bioenergy 5(2) (2013) 62—79. https://modernscientificpress.com/Journals/ViewArticle.aspx?gkN1Z6Pb60HNQPymfPQlZF418S3XB/1Y/g5SJIrtEz9tnj9x30LtKANTNh7z40/x
J. de Souza Macedo, C.J. Nivan B. da, E.A. Luis, S.V. Eunice F. da, R.C. Antonio, F.G. Iara de, V.C. Neftali L., S.B. Ledjane, J. Colloid Interface Sci. 298 (2006) 515—522. https://doi.org/10.1016/j.jcis.2006.01.021
L. Yi, W. Jintao, Z. Yian, W. Aiqin, Chem. Eng. J. 184 (2012) 248—255.https://doi.org/10.1016/j.cej.2012.01.049
D.L. Pavia, G.M. Lampman, G.S. Kriz, Introduction to Spectroscopy: A Guide for Students of Organic Chemistry, 3rd Ed, Thomson Learning, (2001), p. 26. https://www.hdki.hr/_download/repository/Pavia-Introduction-to-Spectroscopy%5B1%5D.pdf
S. Keshk, W. Suwinarti, K. Sameshima, Carbohydr. Polym. 65 (2006) 202—206. https://doi.org/10.1016/j.carbpol.2006.01.005
A. Saeed, M. Sharif, M. Iqbal, J. Hazard. Mater. 179(1-3) (2010) 564—572. https://doi.org/10.1016/j.jhazmat.2010.03.041
R. Ahmad, J. Hazard. Mater. 171(1-3) (2009) 767—773. https://doi.org/10.1016/j.jhazmat.2009.06.060
S. Iman, S. Mahboube, S. Abolfazl, H. Mohsen, H. Saeed, Arab. J. Sci. Eng. 41 (2016) 2611—2621. https://doi.org/10.1007/s13369-016-2109-3
M. Chandrasekaran, M.S. Vaiyazhipalayam, T. Marimuthu, J. Taiwan Inst. Chem. Eng. 63 (2016) 354—362. https://doi.org/10.1016/j.jtice.2016.03.034
A.L. Prasad, T. Shanti, Sustain. Environ. Res. 22(2) (2012) 113—122.
S.H. Maron, C.F. Prutton, Principles of Physical Chemistry, MacMiillan Publishing Co., (1972), p. 813.
R.A. Latour, J. Biomed. Mater. Res. A: 103A: (2015) 949—958. https://doi.org/10.1002/jbm.a.35235
M.B. Yahia, Y.B. Torkia, S. Knani, M.A. Hachicha, M. Khalfaoui, A.B. Lamine, Adsorpt. Sci. Technol. 31(4) (2013) 341—357. https://doi.org/10.1260/0263-6174.31.4.341
R.P.H, Gasser, An Introduction to Chemisorption and Catalysis by Metals, Oxford University Press, (1987), p. 12. ISBN 0198552718
N. Ayawei, S.S. Angaye, D. Wankasi, E.D. Dikio, Open J. Phys. Chem. 5(3) (2015a) 56—70. https://doi.org/10.4236/ojpc.2015.53007
N. Ayawei, A.T. Ekubo, D. Wankasi, E.D. Dikio, Orient. J. Chem. 31(30) (2015b) 1307—1318. http://dx.doi.org/10.13005/ojc/310307
S. Muhammad, T. Hajira, K. Jawariya, H. Uzma, S. Atika, Ultrason. Sonochem. 34 (2017) 600—608. https://doi.org/10.1016/j.ultsonch.2016.06.022
S.R. Shirsath, A.P. Patil, B.A. Bhanvase, S.H. Sonawane, J. Environ. Chem. Eng. 3(2) (2015) 1152—1162. https://doi.org/10.1016/j.jece.2015.04.016
Y. Ho, G. McKay, Process Biochem. 34(5) (1999) 451—465. https://doi.org/10.1016/S0032-9592(98)00112-5
A.D. Becke, J. Chem. Phys. 98(7) (1993) 5648—5652. https://doi.org/10.1063/1.464913
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37(2) (1988) 785—789. https://doi.org/10.1103/physrevb.37.785
J.K. Saha, M.S. Hossain, M.K. Ghosh, Struct. Chem. 30 (2019) 1427—1436. https://doi.org/10.1007/s11224-018-1272-4
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, et. al., Gaussian 16 Rev. C.01, Wallingford, CT (2016).
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.



